Gas Laws - End of Year Chemistry Project
Текст на Статията
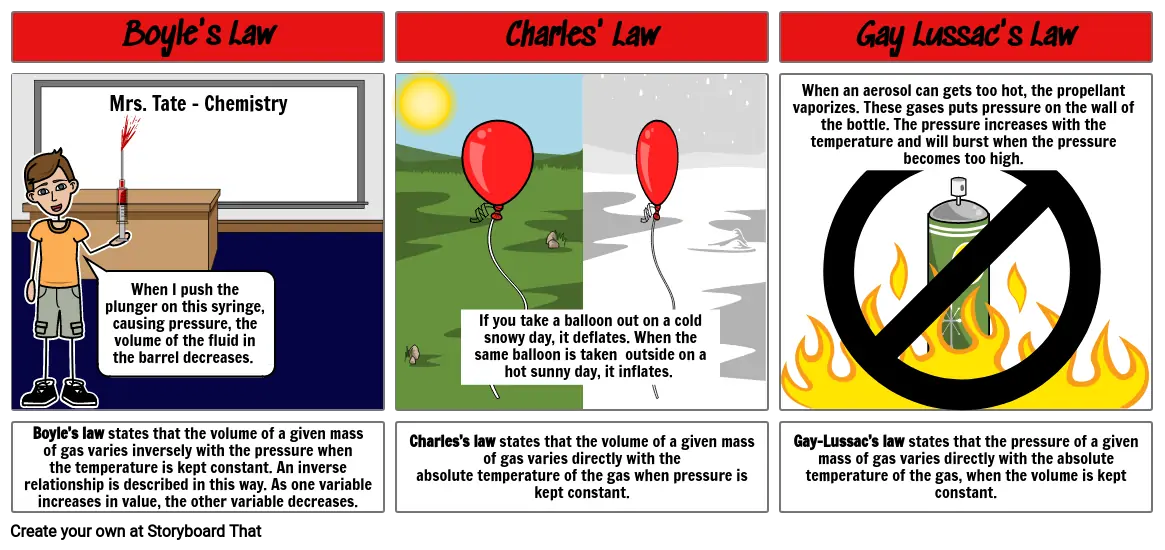
- Boyle's Law
- Mrs. Tate - Chemistry
- When I push the plunger on this syringe, causing pressure, the volume of the fluid in the barrel decreases.
- Charles' Law
- If you take a balloon out on a cold snowy day, it deflates. When the same balloon is taken outside on a hot sunny day, it inflates.
- Gay Lussac's Law
- When an aerosol can gets too hot, the propellant vaporizes. These gases puts pressure on the wall of the bottle. The pressure increases with the temperature and will burst when the pressure becomes too high.
- Boyle’s law states that the volume of a given mass of gas varies inversely with the pressure when the temperature is kept constant. An inverse relationship is described in this way. As one variable increases in value, the other variable decreases.
- Charles’s law states that the volume of a given mass of gas varies directly with the absolute temperature of the gas when pressure is kept constant.
- Gay-Lussac’s law states that the pressure of a given mass of gas varies directly with the absolute temperature of the gas, when the volume is kept constant.
Над 30 милиона създадени разкадровки

