What is Atomic Mass?
Storyboard Text
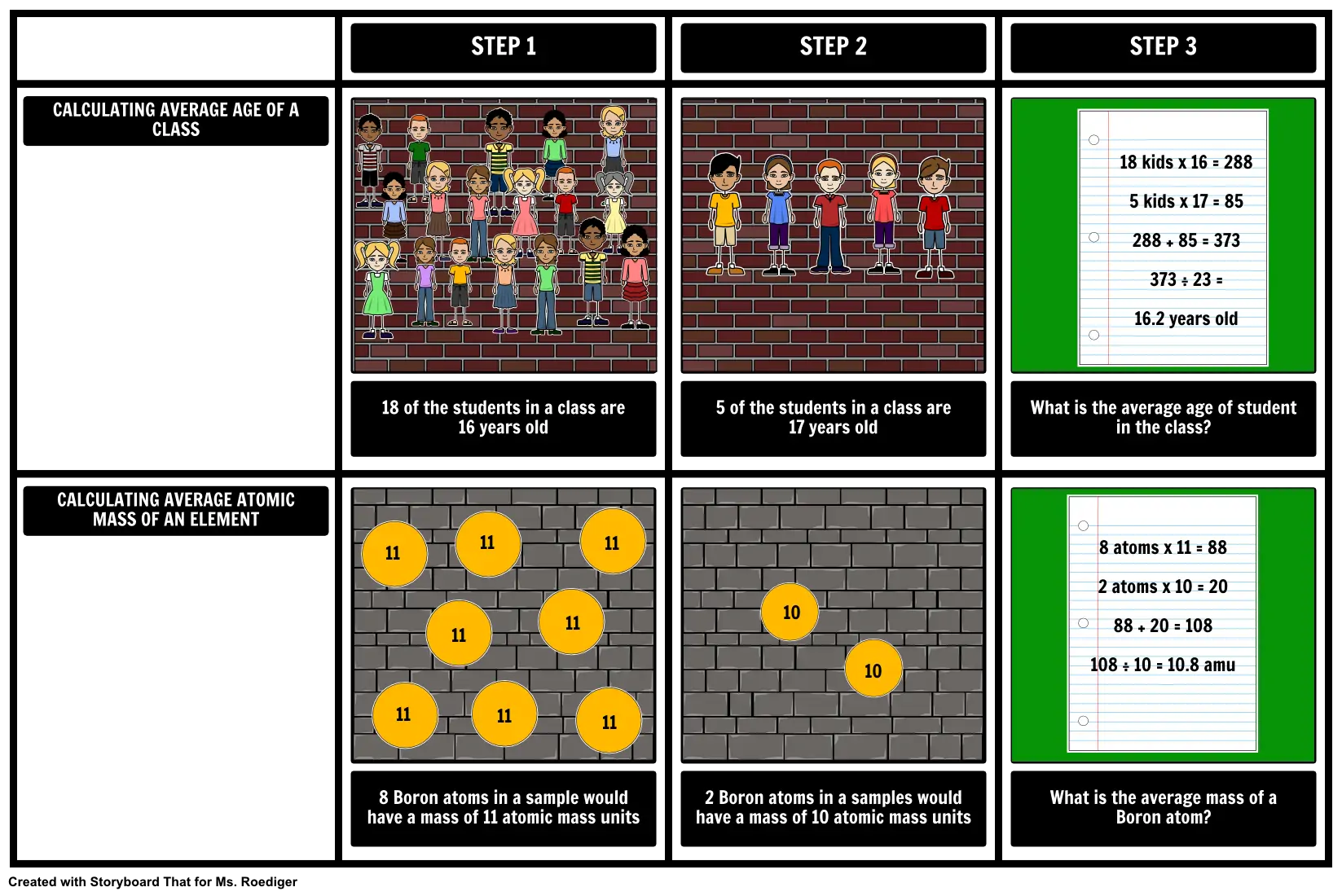
- CALCULATING AVERAGE AGE OF A CLASS
- STEP 1
- STEP 2
- STEP 3
- 18 kids x 16 = 288 5 kids x 17 = 85 288 + 85 = 373 373 ÷ 23 = 16.2 years old
- CALCULATING AVERAGE ATOMIC MASS OF AN ELEMENT
- 18 of the students in a class are 16 years old
- 11
- 11
- 11
- 5 of the students in a class are 17 years old
- What is the average age of student in the class?
- 8 atoms x 11 = 88 2 atoms x 10 = 20 88 + 20 = 108 108 ÷ 10 = 10.8 amu
- 8 Boron atoms in a sample would have a mass of 11 atomic mass units
- 11
- 11
- 11
- 11
- 11
- 2 Boron atoms in a samples would have a mass of 10 atomic mass units
- 10
- 10
- What is the average mass of a Boron atom?
Vytvořeno více než 30 milionů Storyboardů

