Periodic properties of elements
Storyboard Text
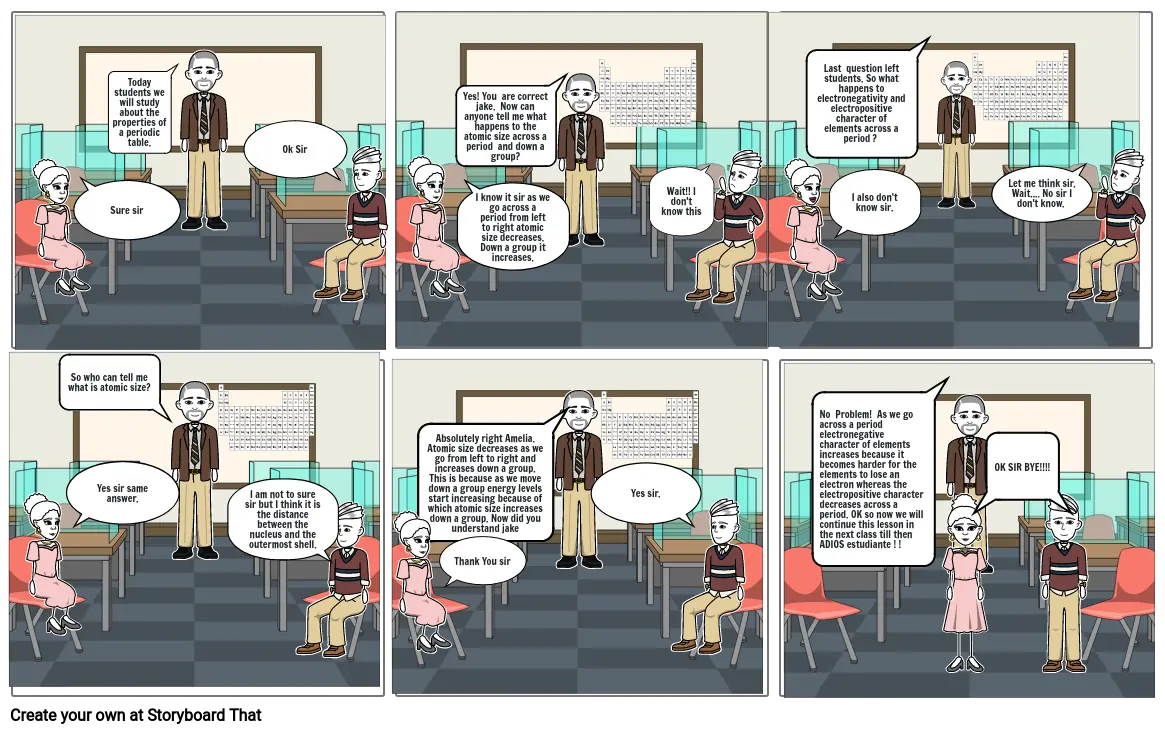
- Sure sir
- Today students we will study about the properties of a periodic table.
- Ok Sir
- Yes! You are correct jake. Now can anyone tell me what happens to the atomic size across a period and down a group?
- I know it sir as we go across a period from left to right atomic size decreases. Down a group it increases.
- Wait!! I don't know this
- Last question left students. So what happens to electronegativity and electropositive character of elements across a period ?
- I also don't know sir.
- Let me think sir. Wait.... No sir I don't know.
- So who can tell me what is atomic size?
- Yes sir same answer.
- I am not to sure sir but I think it is the distance between the nucleus and the outermost shell.
- Absolutely right Amelia. Atomic size decreases as we go from left to right and increases down a group. This is because as we move down a group energy levels start increasing because of which atomic size increases down a group. Now did you understand jake
- Thank You sir
- Yes sir.
- No Problem! As we go across a period electronegative character of elements increases because it becomes harder for the elements to lose an electron whereas the electropositive character decreases across a period. OK so now we will continue this lesson in the next class till then ADIOS estudiante ! !
- OK SIR BYE!!!!
Vytvořeno více než 30 milionů Storyboardů
K Vyzkoušení Není Potřeba Žádné Stahování, Žádná Kreditní Karta a Žádné Přihlášení!
