Chem
Storyboard Text
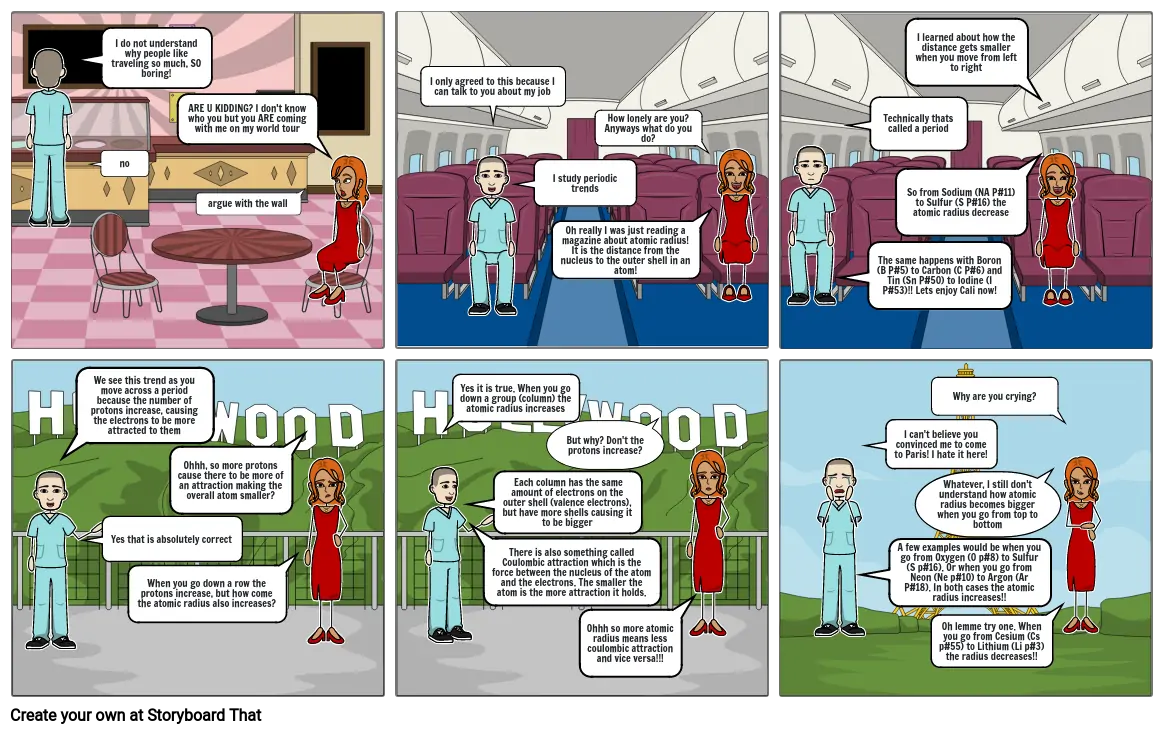
- I do not understand why people like traveling so much, SO boring!
- no
- ARE U KIDDING? I don't know who you but you ARE coming with me on my world tour
- argue with the wall
- I only agreed to this because I can talk to you about my job
- I study periodic trends
- Oh really I was just reading a magazine about atomic radius! It is the distance from the nucleus to the outer shell in an atom!
- How lonely are you? Anyways what do you do?
- The same happens with Boron (B P#5) to Carbon (C P#6) and Tin (Sn P#50) to Iodine (I P#53)!! Lets enjoy Cali now!
- Technically thats called a period
- So from Sodium (NA P#11) to Sulfur (S P#16) the atomic radius decrease
- I learned about how the distance gets smaller when you move from left to right
- We see this trend as you move across a period because the number of protons increase, causing the electrons to be more attracted to them
- Yes that is absolutely correct
- When you go down a row the protons increase, but how come the atomic radius also increases?
- Ohhh, so more protons cause there to be more of an attraction making the overall atom smaller?
- Yes it is true. When you go down a group (column) the atomic radius increases
- Each column has the same amount of electrons on the outer shell (valence electrons), but have more shells causing it to be bigger
- There is also something called Coulombic attraction which is the force between the nucleus of the atom and the electrons. The smaller the atom is the more attraction it holds.
- But why? Don't the protons increase?
- Ohhh so more atomic radius means less coulombic attraction and vice versa!!!
- A few examples would be when you go from Oxygen (0 p#8) to Sulfur (S p#16). Or when you go from Neon (Ne p#10) to Argon (Ar P#18). In both cases the atomic radius increases!!
- I can't believe you convinced me to come to Paris! I hate it here!
- Whatever, I still don't understand how atomic radius becomes bigger when you go from top to bottom
- Oh lemme try one. When you go from Cesium (Cs p#55) to Lithium (Li p#3) the radius decreases!!
- Why are you crying?
Peste 30 de milioane de Storyboard-uri create
Fără Descărcări, Fără Card de Credit și Fără Autentificare Pentru a Încerca!
