Hernandez, Mark Rainier R.
Storyboard Text
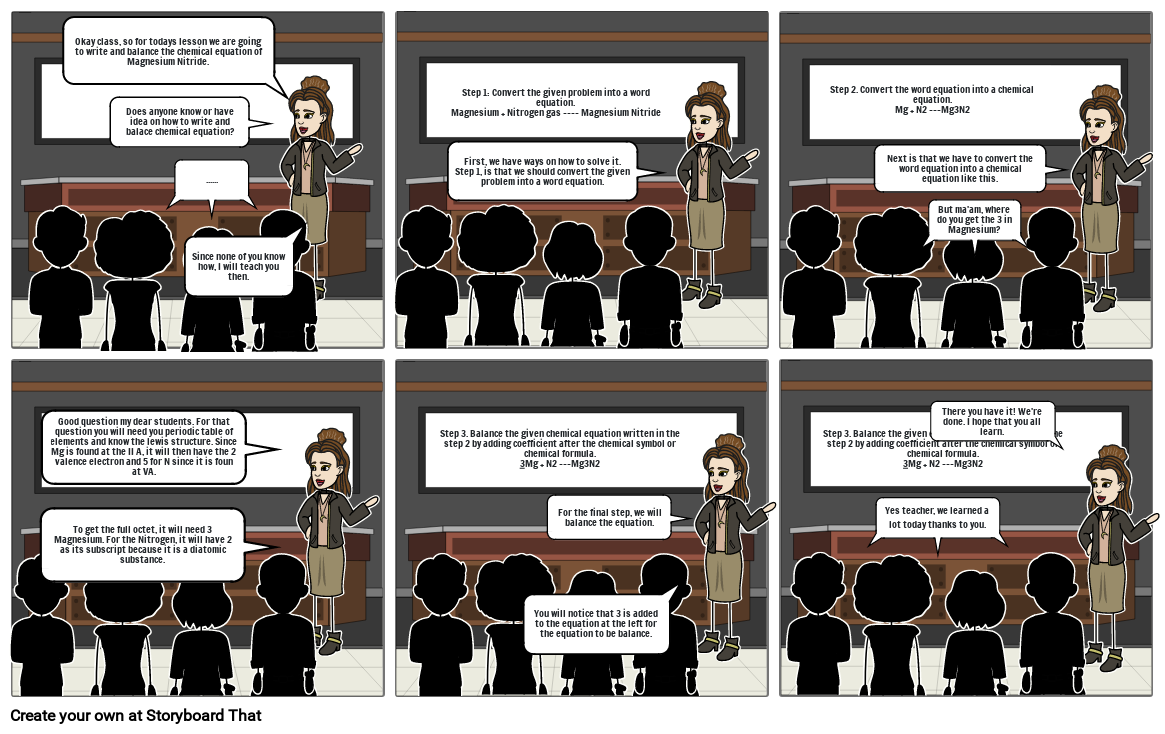
- Okay class, so for todays lesson we are going to write and balance the chemical equation of Magnesium Nitride.
- ......
- Since none of you know how, I will teach you then.
- Does anyone know or have idea on how to write and balace chemical equation?
- Step 1: Convert the given problem into a word equation.Magnesium + Nitrogen gas ---- Magnesium Nitride
- First, we have ways on how to solve it. Step 1, is that we should convert the given problem into a word equation.
- Step 2. Convert the word equation into a chemical equation.Mg + N2 ---Mg3N2
- But ma'am, where do you get the 3 in Magnesium?
- Next is that we have to convert the word equation into a chemical equation like this.
- To get the full octet, it will need 3 Magnesium. For the Nitrogen, it will have 2 as its subscript because it is a diatomic substance.
- Good question my dear students. For that question you will need you periodic table of elements and know the lewis structure. Since Mg is found at the II A, it will then have the 2 valence electron and 5 for N since it is foun at VA.
- Step 3. Balance the given chemical equation written in the step 2 by adding coefficient after the chemical symbol or chemical formula.3Mg + N2 ---Mg3N2
- You will notice that 3 is added to the equation at the left for the equation to be balance.
- For the final step, we will balance the equation.
- Step 3. Balance the given chemical equation written in the step 2 by adding coefficient after the chemical symbol or chemical formula.3Mg + N2 ---Mg3N2
- Yes teacher, we learned a lot today thanks to you.
- There you have it! We're done. I hope that you all learn.
Peste 30 de milioane de Storyboard-uri create
Fără Descărcări, Fără Card de Credit și Fără Autentificare Pentru a Încerca!
