Parts of the Atom
Storyboard Popis
What is an atom made of? What makes elements different from one another? This storyboard helps students understand the answers to these questions.
Text z Príbehu
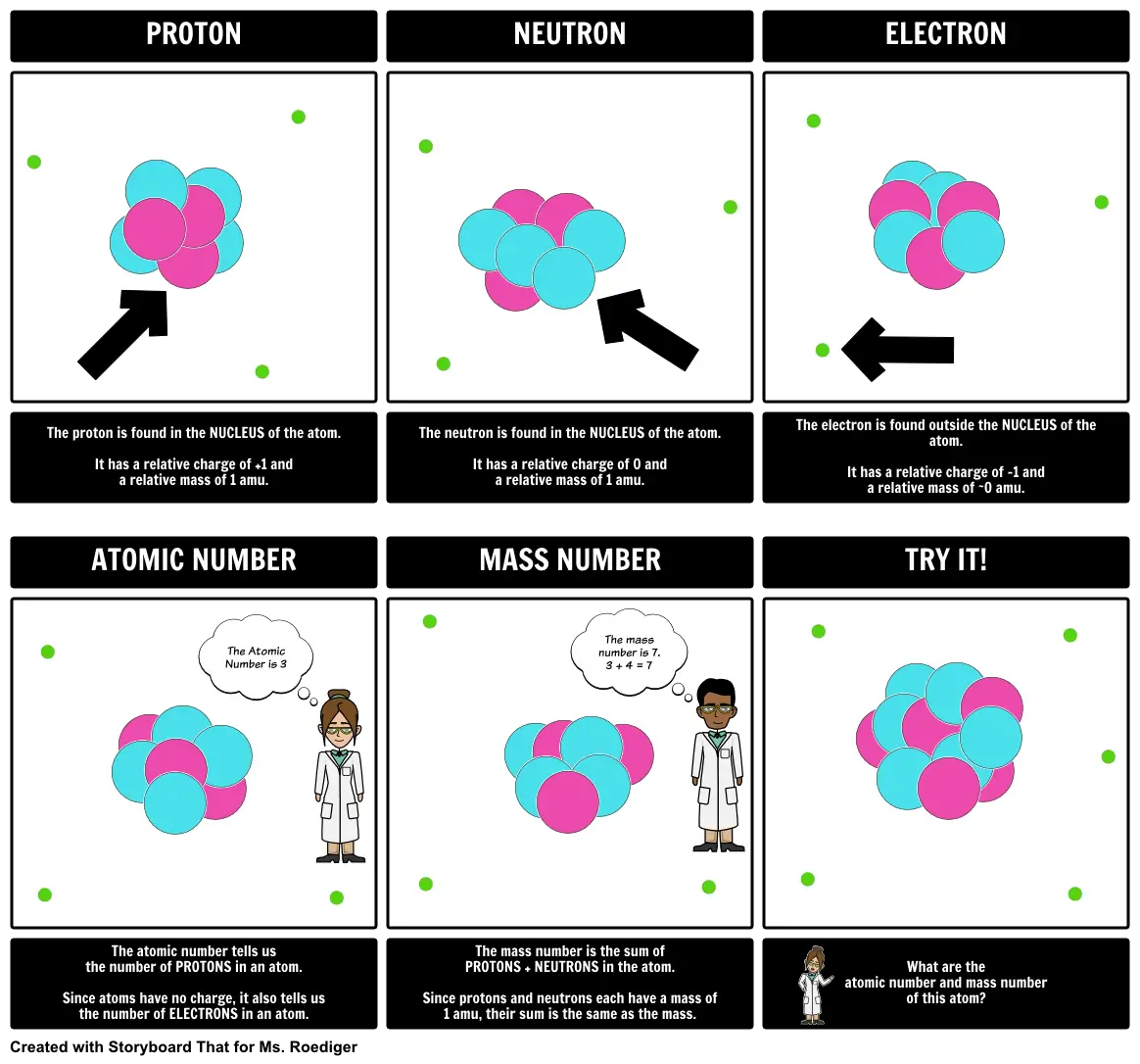
- PROTON
- NEUTRON
- ELECTRON
- The proton is found in the NUCLEUS of the atom. It has a relative charge of +1 and a relative mass of 1 amu.
- ATOMIC NUMBER
- The Atomic Number is 3
- The neutron is found in the NUCLEUS of the atom. It has a relative charge of 0 and a relative mass of 1 amu.
- MASS NUMBER
- The mass number is 7. 3 + 4 = 7
- The electron is found outside the NUCLEUS of the atom. It has a relative charge of -1 and a relative mass of ~0 amu.
- TRY IT!
- The atomic number tells us the number of PROTONS in an atom. Since atoms have no charge, it also tells us the number of ELECTRONS in an atom.
- The mass number is the sum of PROTONS + NEUTRONS in the atom. Since protons and neutrons each have a mass of 1 amu, their sum is the same as the mass.
- What are the atomic number and mass number of this atom?
Bolo vytvorených viac ako 30 miliónov storyboardov
Na Vyskúšanie nie je Potrebné Žiadne Sťahovanie, Žiadna Kreditná Karta a Žiadne Prihlásenie!
