Balancing Chemical Equations
Text z Príbehu
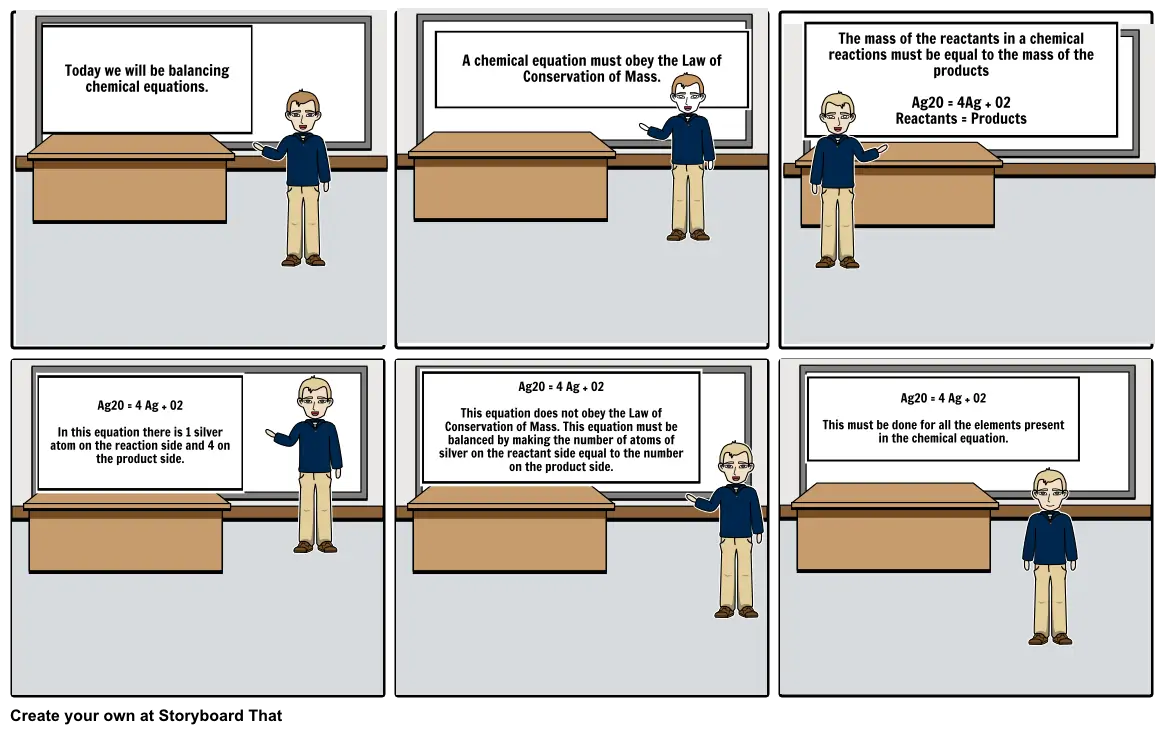
- Today we will be balancing chemical equations.
- A chemical equation must obey the Law of Conservation of Mass.
- The mass of the reactants in a chemical reactions must be equal to the mass of the products Ag2O = 4Ag + O2 Reactants = Products
- Ag2O = 4 Ag + O2 In this equation there is 1 silver atom on the reaction side and 4 on the product side.
- Ag2O = 4 Ag + O2 This equation does not obey the Law of Conservation of Mass. This equation must be balanced by making the number of atoms of silver on the reactant side equal to the number on the product side.
- Ag2O = 4 Ag + O2 This must be done for all the elements present in the chemical equation.
Bolo vytvorených viac ako 30 miliónov storyboardov
Na Vyskúšanie nie je Potrebné Žiadne Sťahovanie, Žiadna Kreditná Karta a Žiadne Prihlásenie!
