law of conservation
Storyboard Text
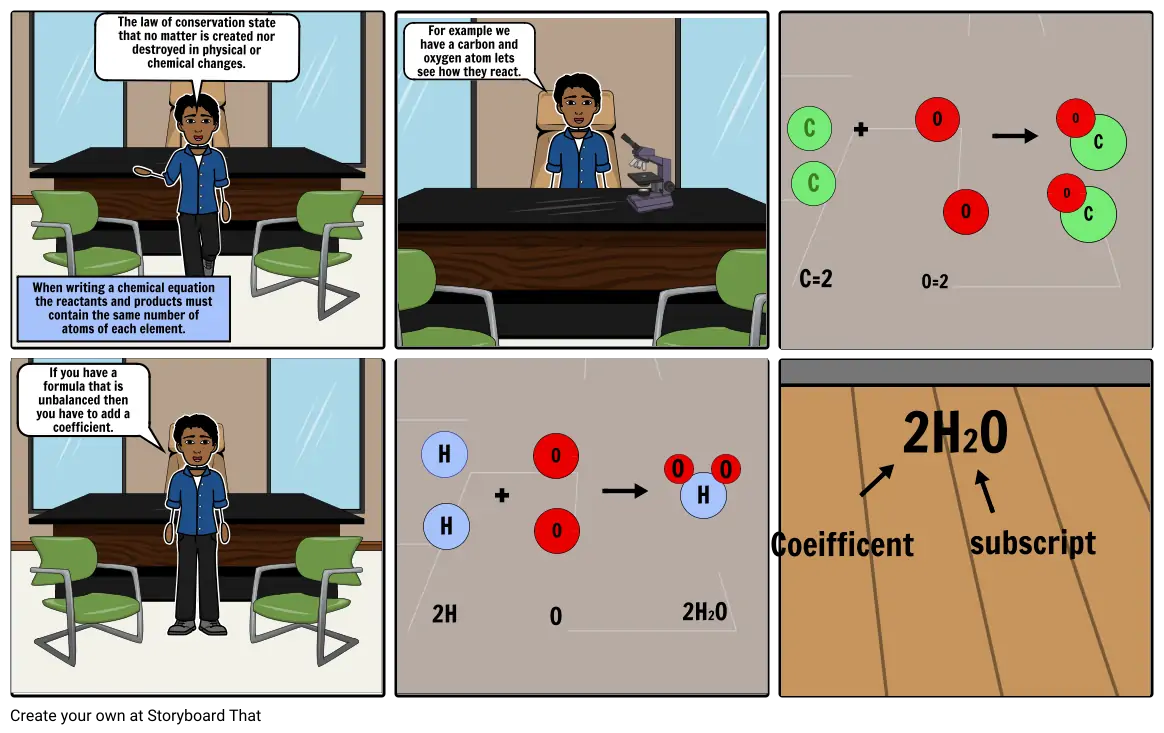
- When writing a chemical equation the reactants and products must contain the same number of atoms of each element.
- The law of conservation state that no matter is created nor destroyed in physical or chemical changes.
- For example we have a carbon and oxygen atom lets see how they react.
- C=2
- C
- C
- +
- O=2
- O
- O
- O
- C
- C
- O
- If you have a formula that is unbalanced then you have to add a coefficient.
- 2H
- H
- H
- +
- O
- O
- O
- 2H2O
- O
- H
- O
- Coeifficent
- 2H2O
- subscript
Over 30 Million Storyboards Created

