Unknown Story
Texto del Guión Gráfico
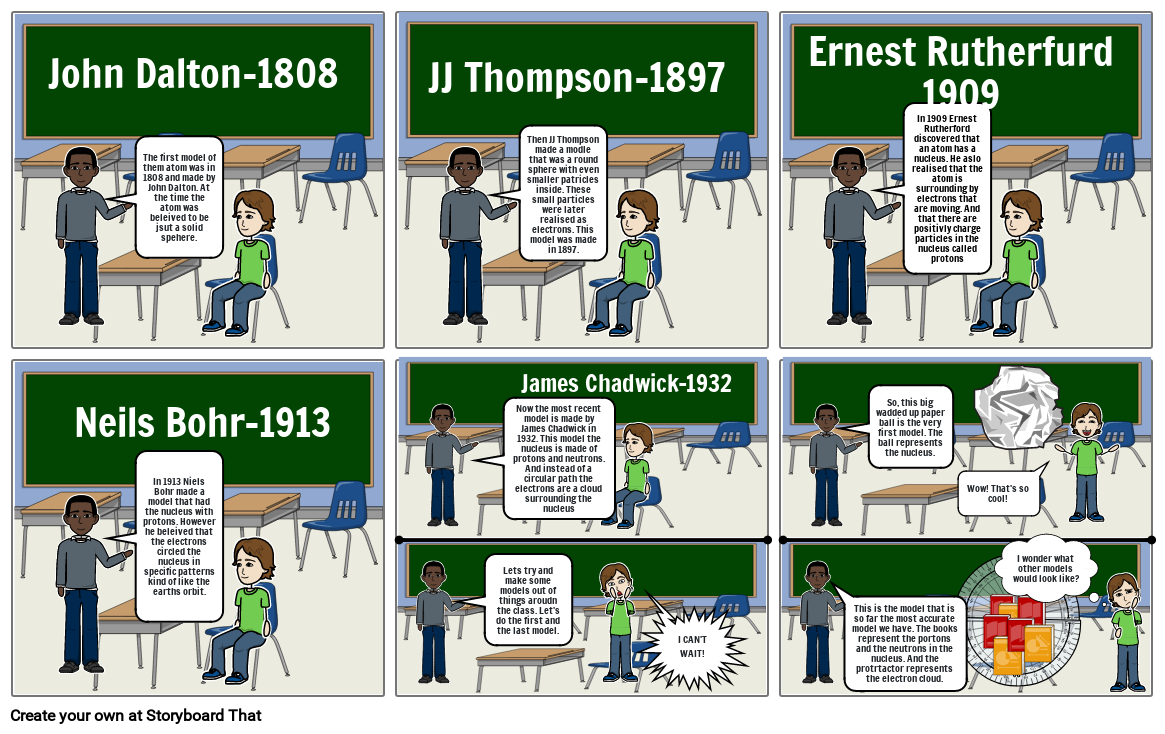
- John Dalton-1808
- The first model of them atom was in 1808 and made by John Dalton. At the time the atom was beleived to be jsut a solid spehere.
- JJ Thompson-1897
- Then JJ Thompson made a modle that was a round sphere with even smaller patricles inside. These small particles were later realised as electrons. This model was made in 1897.
- James Chadwick-1932
- Ernest Rutherfurd 1909
- In 1909 Ernest Rutherford discovered that an atom has a nucleus. He aslo realised that the atom is surrounding by electrons that are moving. And that there are positivly charge particles in the nucleus called protons
- Neils Bohr-1913
- In 1913 Niels Bohr made a model that had the nucleus with protons. However he beleived that the electrons circled the nucleus in specific patterns kind of like the earths orbit.
- Lets try and make some models out of things aroudn the class. Let's do the first and the last model.
- Now the most recent model is made by James Chadwick in 1932. This model the nucleus is made of protons and neutrons. And instead of a circular path the electrons are a cloud surrounding the nucleus
- I CAN'T WAIT!
- This is the model that is so far the most accurate model we have. The books represent the portons and the neutrons in the nucleus. And the protrtactor represents the electron cloud.
- So, this big wadded up paper ball is the very first model. The ball represents the nucleus.
- Wow! That's so cool!
- I wonder what other models would look like?
Más de 30 millones de guiones gráficos creados
¡Sin Descargas, sin Tarjeta de Crédito y sin Necesidad de Iniciar Sesión Para Probar!
