JUAN TEBELIN_Performance Task#1: Comic Strip part 2
Texto del Guión Gráfico
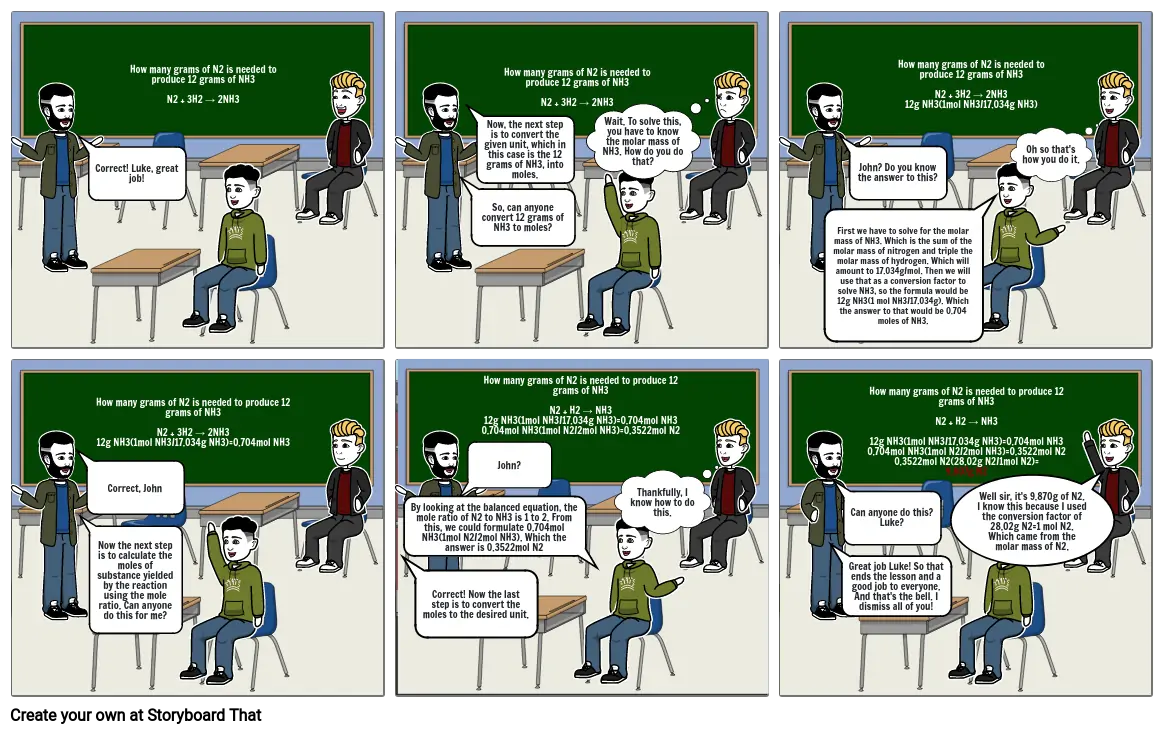
- Correct! Luke, great job!
- How many grams of N2 is needed to produce 12 grams of NH3N2 + 3H2 → 2NH3
- So, can anyone convert 12 grams of NH3 to moles?
- Now, the next step is to convert the given unit, which in this case is the 12 grams of NH3, into moles.
- How many grams of N2 is needed to produce 12 grams of NH3N2 + 3H2 → 2NH3
- Wait. To solve this, you have to know the molar mass of NH3. How do you do that?
- First we have to solve for the molar mass of NH3. Which is the sum of the molar mass of nitrogen and triple the molar mass of hydrogen. Which will amount to 17.034g/mol. Then we will use that as a conversion factor to solve NH3, so the formula would be 12g NH3(1 mol NH3/17.034g). Which the answer to that would be 0.704 moles of NH3.
- John? Do you know the answer to this?
- How many grams of N2 is needed to produce 12 grams of NH3N2 + 3H2 → 2NH312g NH3(1mol NH3/17.034g NH3)
- Oh so that's how you do it.
- Correct, John
- Now the next step is to calculate the moles of substance yielded by the reaction using the mole ratio. Can anyone do this for me?
- How many grams of N2 is needed to produce 12 grams of NH3N2 + 3H2 → 2NH312g NH3(1mol NH3/17.034g NH3)=0.704mol NH3
- Correct! Now the last step is to convert the moles to the desired unit.
- By looking at the balanced equation, the mole ratio of N2 to NH3 is 1 to 2. From this, we could formulate 0.704mol NH3(1mol N2/2mol NH3). Which the answer is 0.3522mol N2
- John?
- How many grams of N2 is needed to produce 12 grams of NH3N2 + H2 → NH312g NH3(1mol NH3/17.034g NH3)=0.704mol NH30.704mol NH3(1mol N2/2mol NH3)=0.3522mol N2
- Thankfully, I know how to do this.
- Great job Luke! So that ends the lesson and a good job to everyone. And that's the bell. I dismiss all of you!
- Can anyone do this? Luke?
- How many grams of N2 is needed to produce 12 grams of NH3N2 + H2 → NH312g NH3(1mol NH3/17.034g NH3)=0.704mol NH30.704mol NH3(1mol N2/2mol NH3)=0.3522mol N20.3522mol N2(28.02g N2/1mol N2)=9.807g N2
- Well sir, it's 9.870g of N2. I know this because I used the conversion factor of 28.02g N2=1 mol N2. Which came from the molar mass of N2.
Más de 30 millones de guiones gráficos creados
¡Sin Descargas, sin Tarjeta de Crédito y sin Necesidad de Iniciar Sesión Para Probar!
