The Development of the Modern Atom
Texto del Guión Gráfico
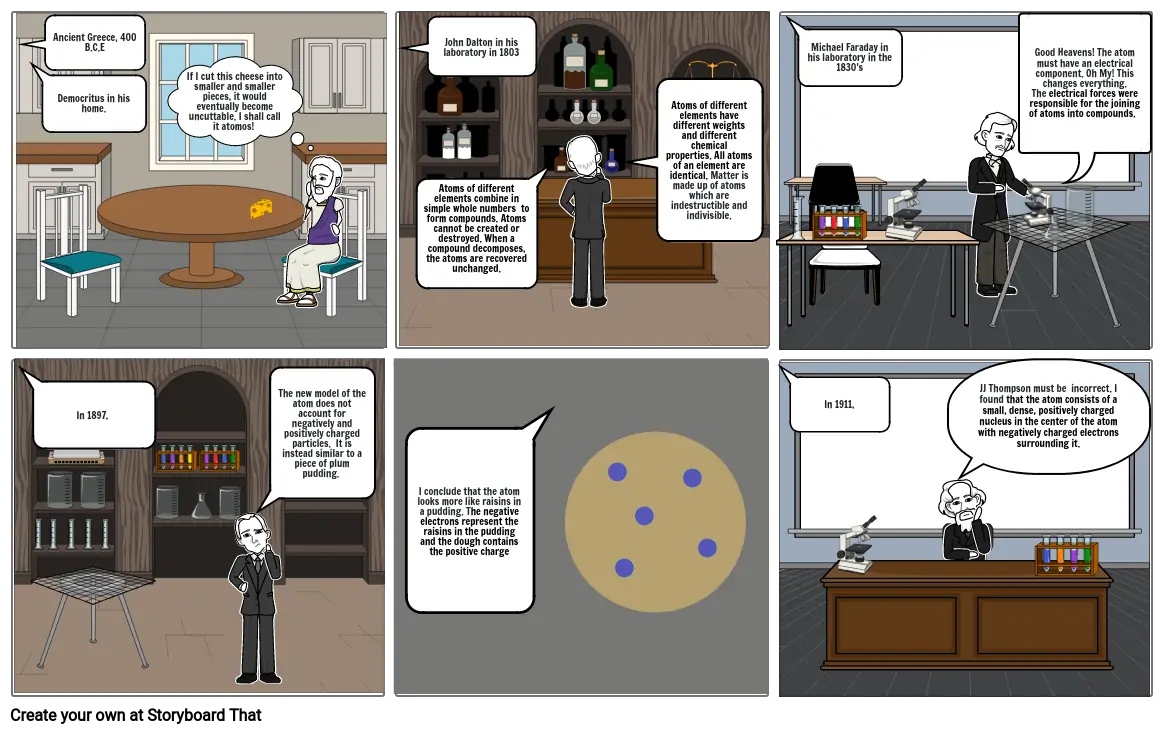
- Ancient Greece, 400 B.C.E
- Democritus in his home.
- If I cut this cheese into smaller and smaller pieces, it would eventually become uncuttable. I shall call it atomos!
- John Dalton in his laboratory in 1803
- Atoms of different elements combine in simple whole numbers to form compounds. Atoms cannot be created or destroyed. When a compound decomposes, the atoms are recovered unchanged.
- Atoms of different elements have different weights and different chemical properties. All atoms of an element are identical. Matter is made up of atoms which are indestructible and indivisible.
- Michael Faraday in his laboratory in the 1830's
- Good Heavens! The atom must have an electrical component. Oh My! This changes everything. The electrical forces were responsible for the joining of atoms into compounds.
- In 1897,
-
- The new model of the atom does not account for negatively and positively charged particles. It is instead similar to a piece of plum pudding.
-
- I conclude that the atom looks more like raisins in a pudding. The negative electrons represent the raisins in the pudding and the dough contains the positive charge
-
-
-
-
-
-
- In 1911,
- JJ Thompson must be incorrect. I found that the atom consists of a small, dense, positively charged nucleus in the center of the atom with negatively charged electrons surrounding it.
Más de 30 millones de guiones gráficos creados
¡Sin Descargas, sin Tarjeta de Crédito y sin Necesidad de Iniciar Sesión Para Probar!
