kittys
טקסט Storyboard
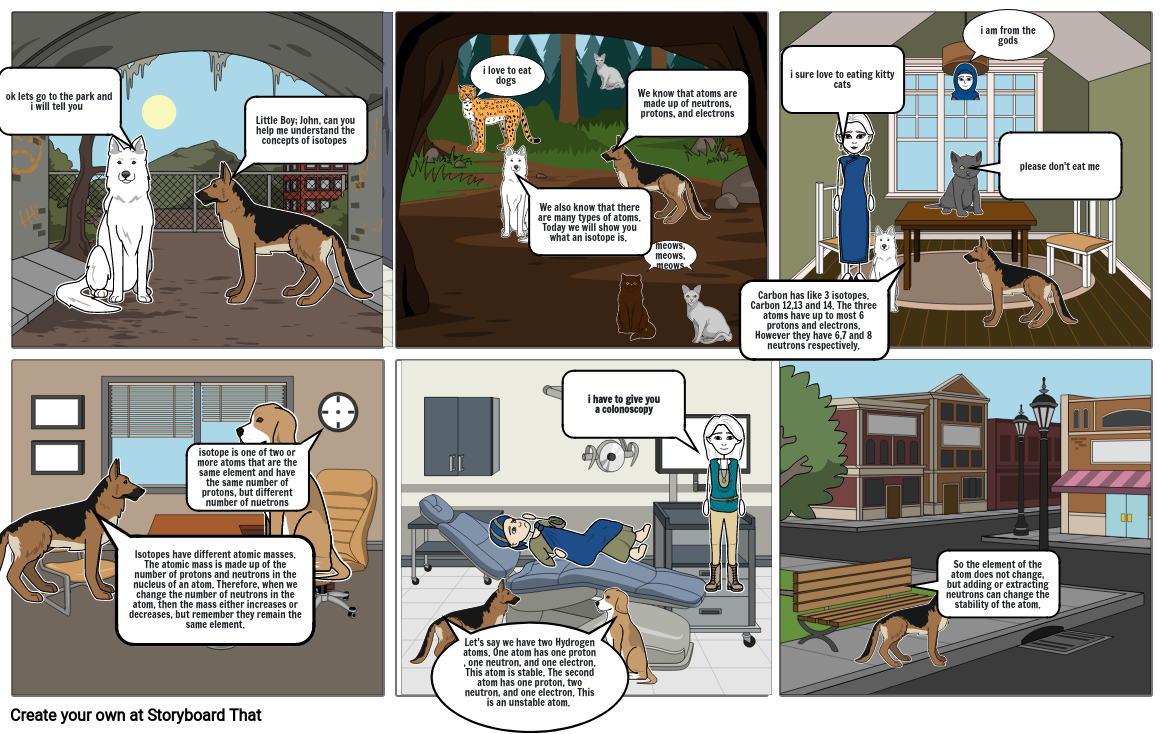
- ok lets go to the park and i will tell you
- Little Boy; John, can you help me understand the concepts of isotopes
- i love to eat dogs
- We also know that there are many types of atoms. Today we will show you what an isotope is.
- We know that atoms are made up of neutrons, protons, and electrons
- meows,meows,meows
- Carbon has like 3 isotopes. Carbon 12,13 and 14. The three atoms have up to most 6 protons and electrons. However they have 6,7 and 8 neutrons respectively.
- i sure love to eating kitty cats
- i am from the gods
- please don't eat me
- isotope is one of two or more atoms that are the same element and have the same number of protons, but different number of nuetrons
- Isotopes have different atomic masses. The atomic mass is made up of the number of protons and neutrons in the nucleus of an atom. Therefore, when we change the number of neutrons in the atom, then the mass either increases or decreases, but remember they remain the same element.
- i have to give you a colonoscopy
- So the element of the atom does not change, but adding or extracting neutrons can change the stability of the atom.
- Let's say we have two Hydrogen atoms. One atom has one proton , one neutron, and one electron. This atom is stable. The second atom has one proton, two neutron, and one electron. This is an unstable atom.
נוצרו מעל 30 מיליון לוחות סיפור
אין הורדות, אין כרטיס אשראי ואין צורך בכניסה כדי לנסות!
