Scientist Discovery of Atomic Theory
Storyboard Text
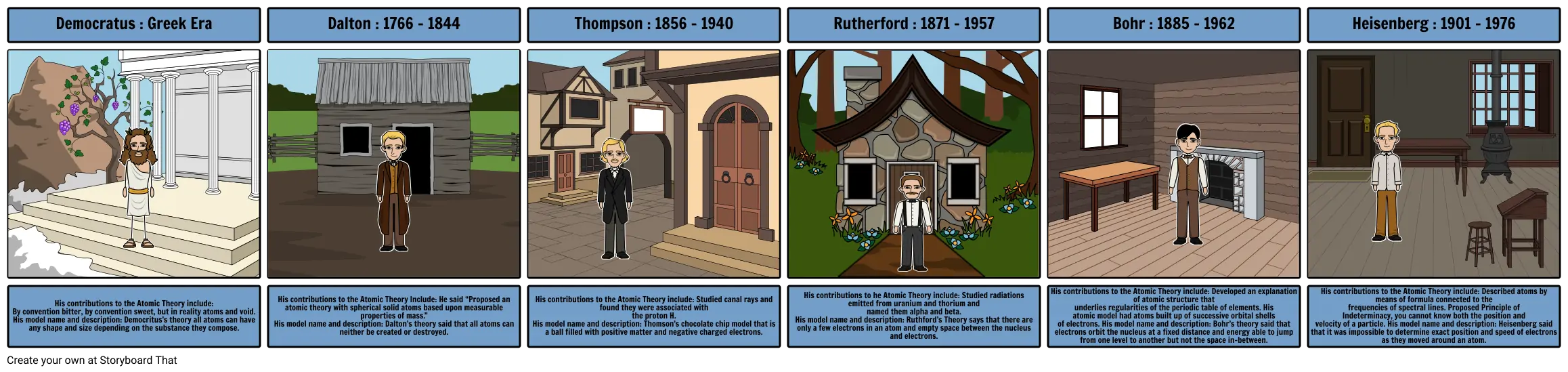
- Democratus : Greek Era
- Dalton : 1766 - 1844
- Thompson : 1856 - 1940
- Rutherford : 1871 - 1957
- Bohr : 1885 - 1962
- Heisenberg : 1901 - 1976
- His contributions to the Atomic Theory include: By convention bitter, by convention sweet, but in reality atoms and void. His model name and description: Democritus’s theory all atoms can have any shape and size depending on the substance they compose.
- His contributions to the Atomic Theory Include: He said “Proposed an atomic theory with spherical solid atoms based upon measurable properties of mass.” His model name and description: Dalton’s theory said that all atoms can neither be created or destroyed.
- His contributions to the Atomic Theory include: Studied canal rays and found they were associated with the proton H. His model name and description: Thomson’s chocolate chip model that is a ball filled with positive matter and negative charged electrons.
- His contributions to he Atomic Theory include: Studied radiations emitted from uranium and thorium and named them alpha and beta. His model name and description: Ruthford’s Theory says that there are only a few electrons in an atom and empty space between the nucleus and electrons.
- His contributions to the Atomic Theory include: Developed an explanation of atomic structure that underlies regularities of the periodic table of elements. His atomic model had atoms built up of successive orbital shells of electrons. His model name and description: Bohr’s theory said that electrons orbit the nucleus at a fixed distance and energy able to jump from one level to another but not the space in-between.
- His contributions to the Atomic Theory include: Described atoms by means of formula connected to the frequencies of spectral lines. Proposed Principle of Indeterminacy, you cannot know both the position and velocity of a particle. His model name and description: Heisenberg said that it was impossible to determine exact position and speed of electrons as they moved around an atom.
Over 30 Million Storyboards Created

