Parts of the Atom
Süžeeskeem Kirjeldus
What is an atom made of? What makes elements different from one another? This storyboard helps students understand the answers to these questions.
Süžeeskeem Tekst
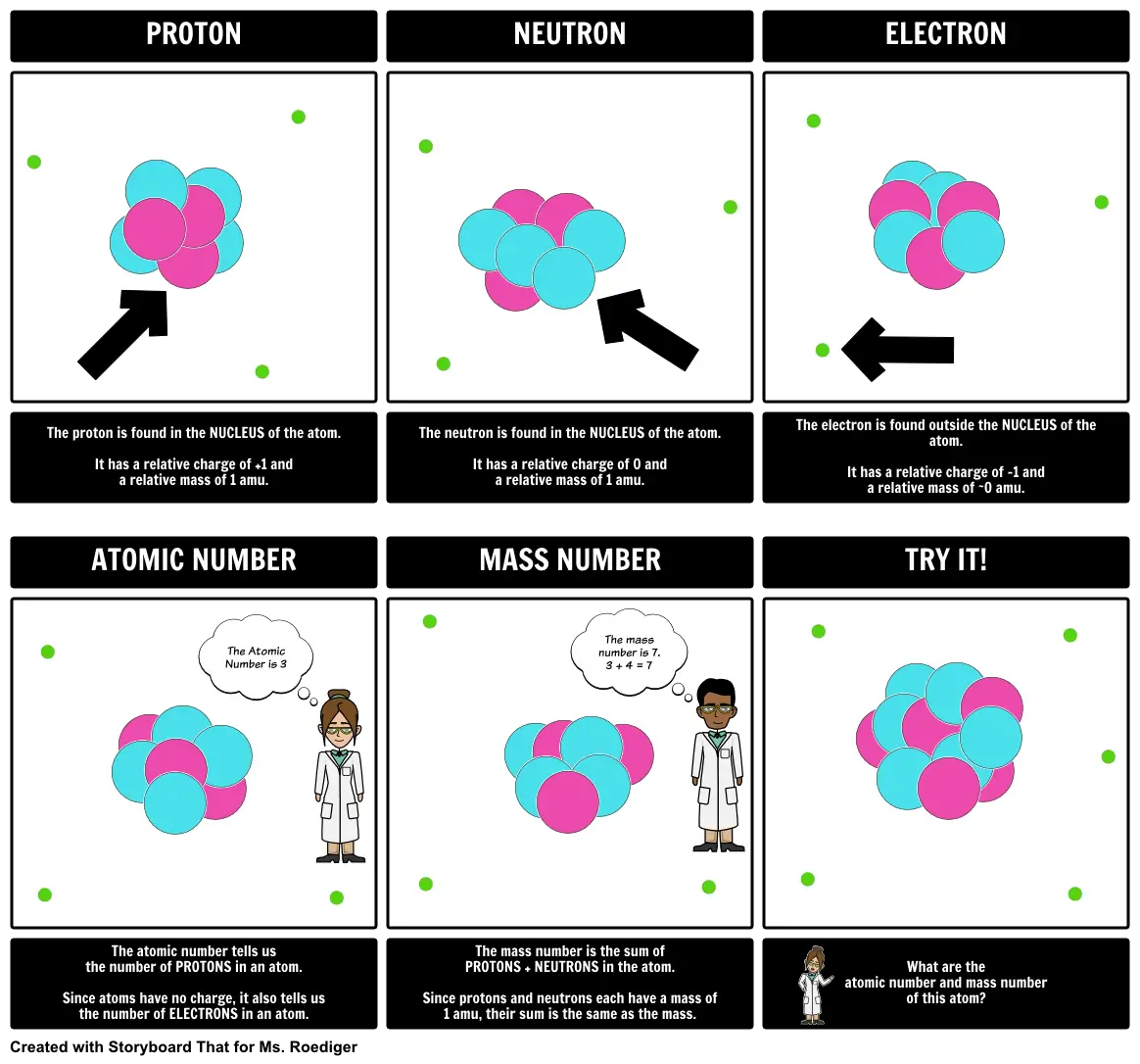
- PROTON
- NEUTRON
- ELECTRON
- The proton is found in the NUCLEUS of the atom. It has a relative charge of +1 and a relative mass of 1 amu.
- ATOMIC NUMBER
- The Atomic Number is 3
- The neutron is found in the NUCLEUS of the atom. It has a relative charge of 0 and a relative mass of 1 amu.
- MASS NUMBER
- The mass number is 7. 3 + 4 = 7
- The electron is found outside the NUCLEUS of the atom. It has a relative charge of -1 and a relative mass of ~0 amu.
- TRY IT!
- The atomic number tells us the number of PROTONS in an atom. Since atoms have no charge, it also tells us the number of ELECTRONS in an atom.
- The mass number is the sum of PROTONS + NEUTRONS in the atom. Since protons and neutrons each have a mass of 1 amu, their sum is the same as the mass.
- What are the atomic number and mass number of this atom?
Loodud üle 30 miljoni süžeeskeemi
Proovimiseks Pole Vaja Allalaadimist, Krediitkaarti ega Sisselogimist!
