Chemistry H.W
Storyboard Tekst
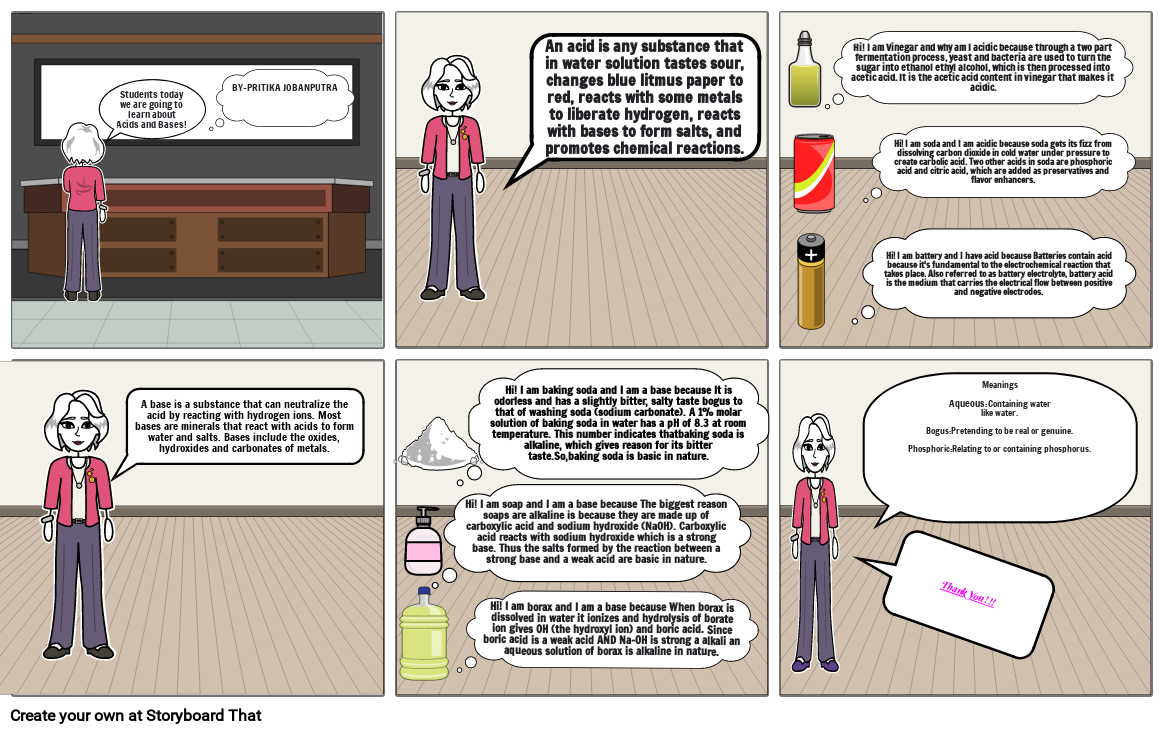
- Students today we are going to learn about Acids and Bases!
- BY-PRITIKA JOBANPUTRA
- An acid is any substance that in water solution tastes sour, changes blue litmus paper to red, reacts with some metals to liberate hydrogen, reacts with bases to form salts, and promotes chemical reactions.
- Hi! I am Vinegar and why am I acidic because through a two part fermentation process, yeast and bacteria are used to turn the sugar into ethanol ethyl alcohol, which is then processed into acetic acid. It is the acetic acid content in vinegar that makes it acidic.
- Hi! I am battery and I have acid because Batteries contain acid because it's fundamental to the electrochemical reaction that takes place. Also referred to as battery electrolyte, battery acid is the medium that carries the electrical flow between positive and negative electrodes.
- Hi! I am soda and I am acidic because soda gets its fizz from dissolving carbon dioxide in cold water under pressure to create carbolic acid. Two other acids in soda are phosphoric acid and citric acid, which are added as preservatives and flavor enhancers.
- A base is a substance that can neutralize the acid by reacting with hydrogen ions. Most bases are minerals that react with acids to form water and salts. Bases include the oxides, hydroxides and carbonates of metals.
- Hi! I am soap and I am a base because The biggest reason soaps are alkaline is because they are made up of carboxylic acid and sodium hydroxide (NaOH). Carboxylic acid reacts with sodium hydroxide which is a strong base. Thus the salts formed by the reaction between a strong base and a weak acid are basic in nature.
- Hi! I am baking soda and I am a base because It is odorless and has a slightly bitter, salty taste bogus to that of washing soda (sodium carbonate). A 1% molar solution of baking soda in water has a pH of 8.3 at room temperature. This number indicates thatbaking soda is alkaline, which gives reason for its bitter taste.So,baking soda is basic in nature.
- Hi! I am borax and I am a base because When borax is dissolved in water it ionizes and hydrolysis of borate ion gives OH (the hydroxyl ion) and boric acid. Since boric acid is a weak acid AND Na-OH is strong a alkali an aqueous solution of borax is alkaline in nature.
- Thank You!!!
- Meanings Aqueous:Containing waterlike water.Bogus:Pretending to be real or genuine.Phosphoric:Relating to or containing phosphorus.
Izrađeno više od 30 milijuna scenarija
Bez Preuzimanja, bez Kreditne Kartice i bez Prijave!
