The Trends of the Periodic Table
Testo Storyboard
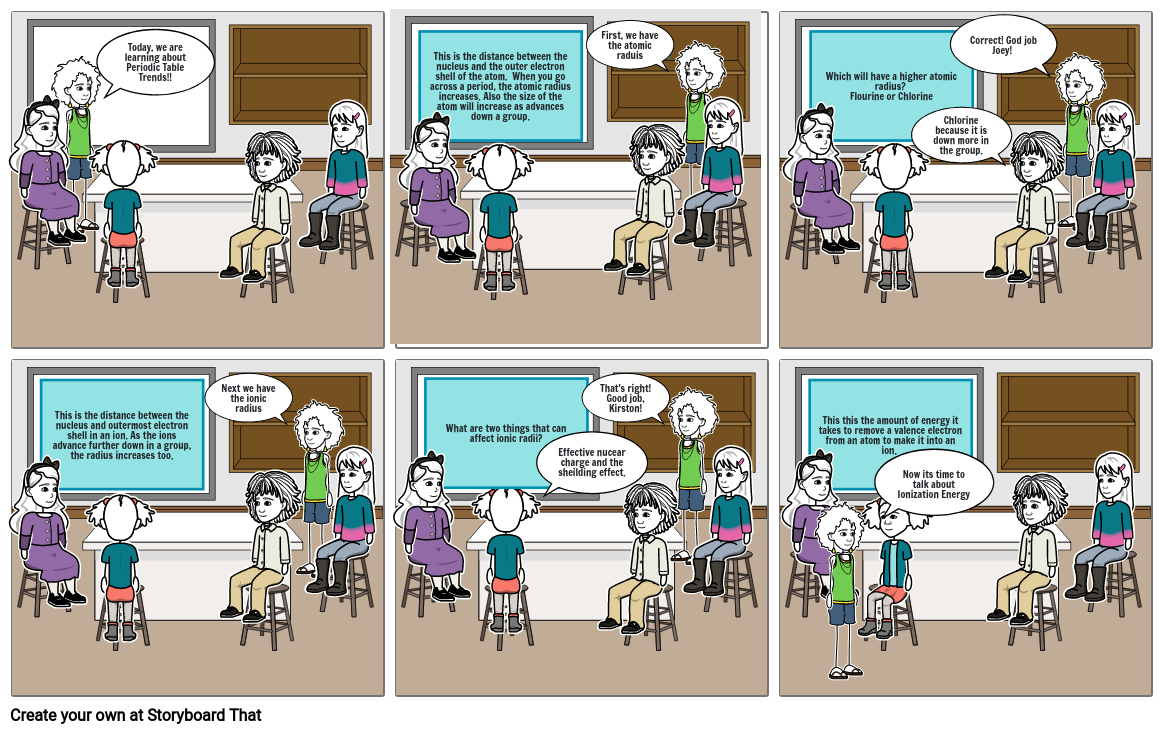
- Today, we are learning about Periodic Table Trends!!
- This is the distance between the nucleus and the outer electron shell of the atom. When you go across a period, the atomic radius increases. Also the size of the atom will increase as advances down a group.
- First, we have the atomic raduis
- Which will have a higher atomic radius? Flourine or Chlorine
- Chlorine because it is down more in the group.
- Correct! God job Joey!
- This is the distance between the nucleus and outermost electron shell in an ion. As the ions advance further down in a group, the radius increases too.
- Next we have the ionic radius
- What are two things that can affect ionic radii?
- Effective nucear charge and the sheilding effect.
- That's right! Good job, Kirston!
- This this the amount of energy it takes to remove a valence electron from an atom to make it into an ion.
- Now its time to talk about Ionization Energy
Oltre 30 milioni di storyboard creati

