Parts of the Atom
Montāžas Apraksts
What is an atom made of? What makes elements different from one another? This storyboard helps students understand the answers to these questions.
Montāžas Teksta
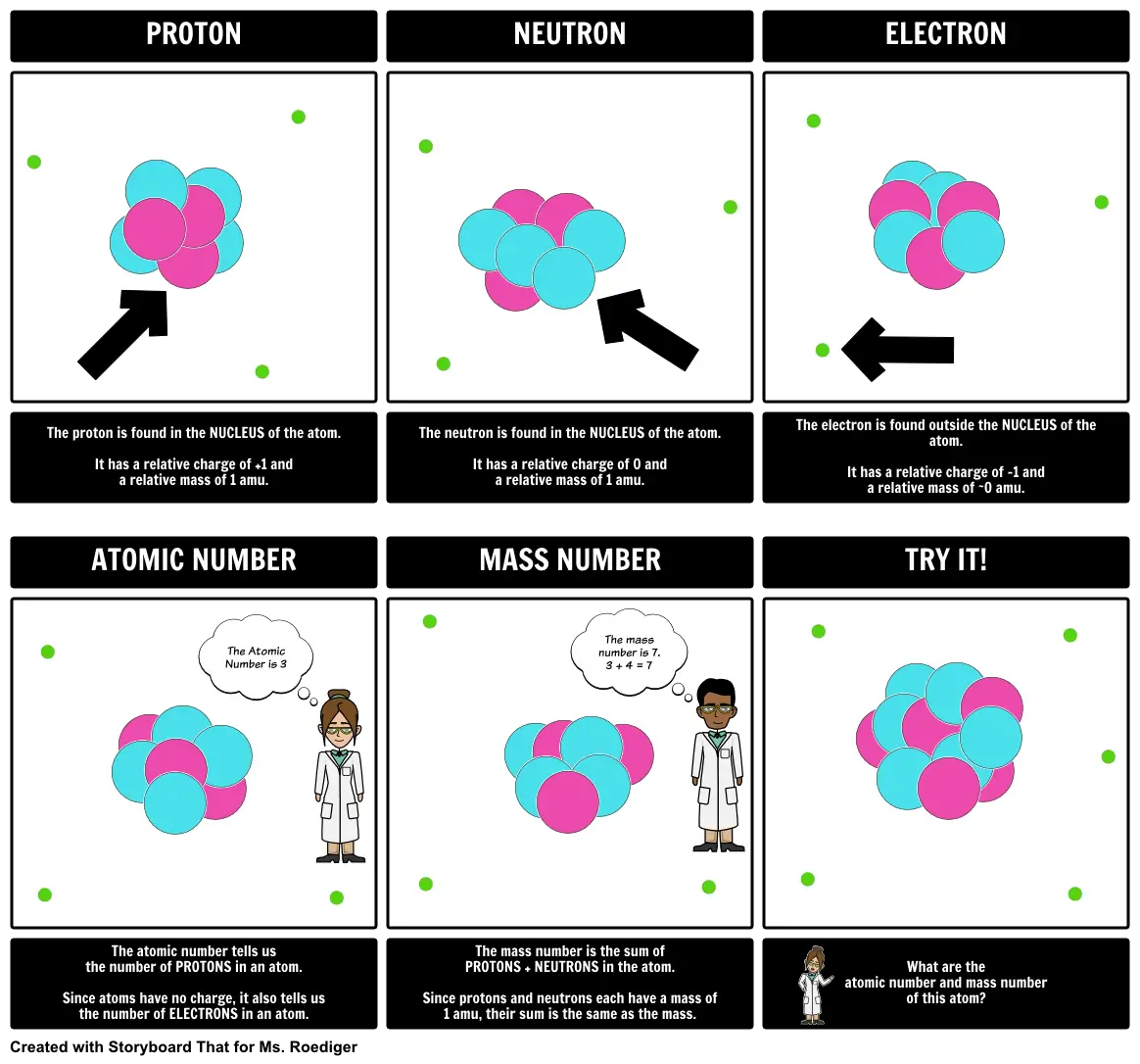
- PROTON
- NEUTRON
- ELECTRON
- The proton is found in the NUCLEUS of the atom. It has a relative charge of +1 and a relative mass of 1 amu.
- ATOMIC NUMBER
- The Atomic Number is 3
- The neutron is found in the NUCLEUS of the atom. It has a relative charge of 0 and a relative mass of 1 amu.
- MASS NUMBER
- The mass number is 7. 3 + 4 = 7
- The electron is found outside the NUCLEUS of the atom. It has a relative charge of -1 and a relative mass of ~0 amu.
- TRY IT!
- The atomic number tells us the number of PROTONS in an atom. Since atoms have no charge, it also tells us the number of ELECTRONS in an atom.
- The mass number is the sum of PROTONS + NEUTRONS in the atom. Since protons and neutrons each have a mass of 1 amu, their sum is the same as the mass.
- What are the atomic number and mass number of this atom?
Izveidoti vairāk nekā 30 miljoni stāstu shēmu
Lai Izmēģinātu, nav Nepieciešama Lejupielāde, nav Kredītkartes un nav Nepieciešama Pieteikšanās!
