Balancing Chemical Equations
Montāžas Teksta
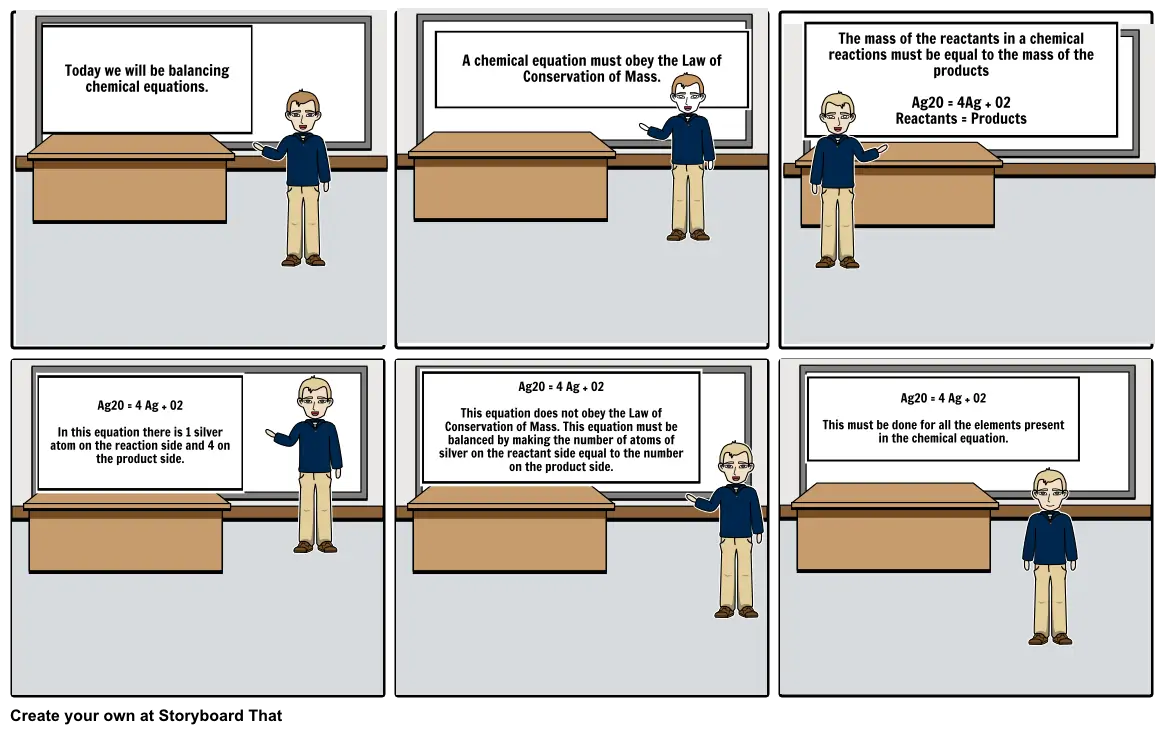
- Today we will be balancing chemical equations.
- A chemical equation must obey the Law of Conservation of Mass.
- The mass of the reactants in a chemical reactions must be equal to the mass of the products Ag2O = 4Ag + O2 Reactants = Products
- Ag2O = 4 Ag + O2 In this equation there is 1 silver atom on the reaction side and 4 on the product side.
- Ag2O = 4 Ag + O2 This equation does not obey the Law of Conservation of Mass. This equation must be balanced by making the number of atoms of silver on the reactant side equal to the number on the product side.
- Ag2O = 4 Ag + O2 This must be done for all the elements present in the chemical equation.
Izveidoti vairāk nekā 30 miljoni stāstu shēmu
Lai Izmēģinātu, nav Nepieciešama Lejupielāde, nav Kredītkartes un nav Nepieciešama Pieteikšanās!
