Unknown Story
Storyboard Text
- ALL ABOUT ATOMIC RADIUS
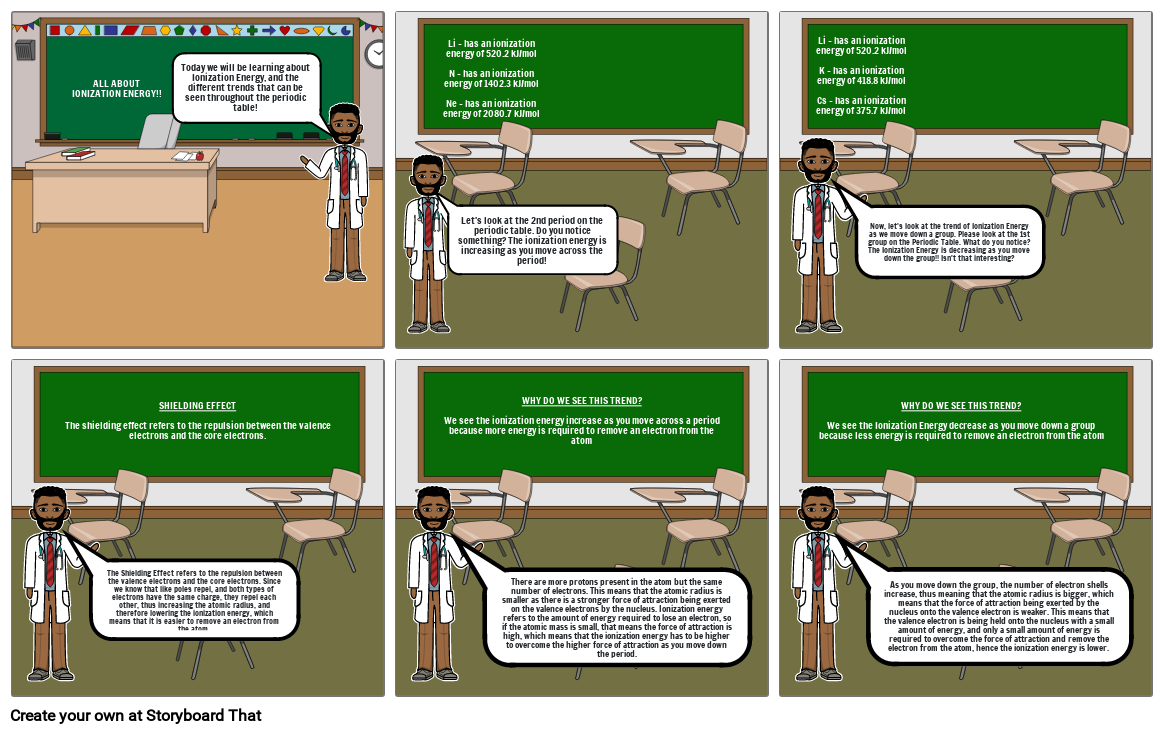
- ALL ABOUT IONIZATION ENERGY!!
- Today we will be learning about Atomic Radius, and the different trends that can be seen throughout the period table!
- Today we will be learning about Ionization Energy, and the different trends that can be seen throughout the periodic table!
- Let's look at the 2nd period on the periodic table. Do you notice something? The ionization energy is increasing as you move across the period!
- Li - has an ionization energy of 520.2 kJ/molN - has an ionization energy of 1402.3 kJ/molNe - has an ionization energy of 2080.7 kJ/mol
- Let's look at the 2nd Period on the periodic table. Do you notice something? The atomic radius is decreasing as you move across the period!
- Li - has an ionization energy of 520.2 kJ/molK - has an ionization energy of 418.8 kJ/molCs - has an ionization energy of 375.7 kJ/mol
- Now, let's look at the trend of Ionization Energy as we move down a group. Please look at the 1st group on the Periodic Table. What do you notice? The Ionization Energy is decreasing as you move down the group!! Isn't that interesting?
- SHIELDING EFFECTThe shielding effect refers to the repulsion between the valence electrons and the core electrons.
- The Shielding Effect refers to the repulsion between the valence electrons and the core electrons. Since we know that like poles repel, and both types of electrons have the same charge, they repel each other, thus increasing the atomic radius, and therefore lowering the ionization energy, which means that it is easier to remove an electron from the atom.
- WHY DO WE SEE THIS TREND?We see the ionization energy increase as you move across a period because more energy is required to remove an electron from the atom
- There are more protons present in the atom but the same number of electrons. This means that the atomic radius is smaller as there is a stronger force of attraction being exerted on the valence electrons by the nucleus. Ionization energy refers to the amount of energy required to lose an electron, so if the atomic mass is small, that means the force of attraction is high, which means that the ionization energy has to be higher to overcome the higher force of attraction as you move down the period.
- WHY DO WE SEE THIS TREND?We see the Ionization Energy decrease as you move down a group because less energy is required to remove an electron from the atom
- As you move down the group, the number of electron shells increase, thus meaning that the atomic radius is bigger, which means that the force of attraction being exerted by the nucleus onto the valence electron is weaker. This means that the valence electron is being held onto the nucleus with a small amount of energy, and only a small amount of energy is required to overcome the force of attraction and remove the electron from the atom, hence the ionization energy is lower.
Over 30 Million Storyboards Created

