Chem Alchamey Lab
Storyboard Text
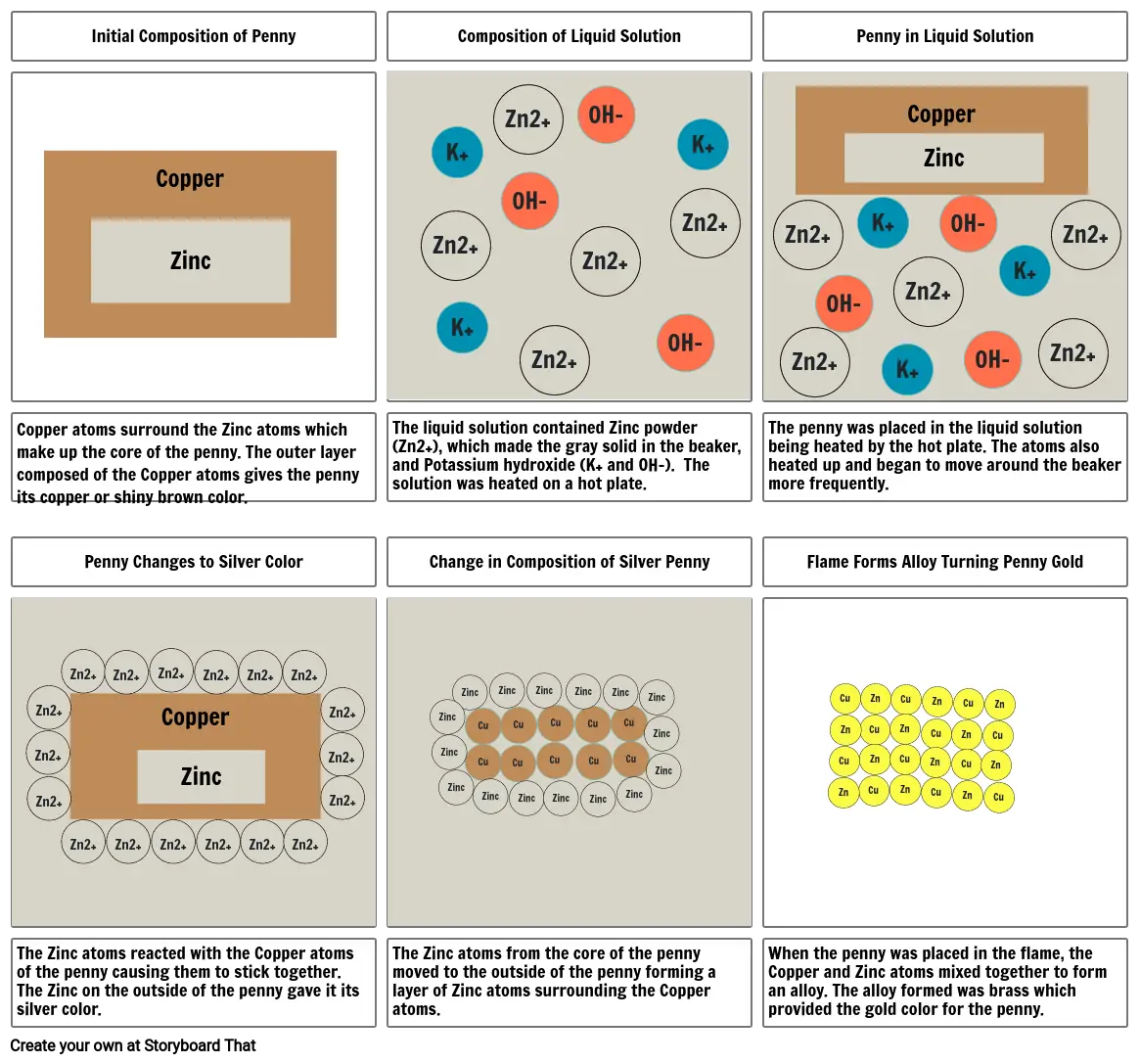
- Initial Composition of Penny
- Copper
- Zinc
- Composition of Liquid Solution
- Zn2+
- K+
- K+
- Zn2+
- OH-
- Zn2+
- Zn2+
- OH-
- OH-
- Zn2+
- K+
- Penny in Liquid Solution
- Zn2+
- Zn2+
- Copper
- OH-
- Zinc
- K+
- Zn2+
- OH-
- OH-
- K+
- Zn2+
- Zn2+
- Copper atoms surround the Zinc atoms which make up the core of the penny. The outer layer composed of the Copper atoms gives the penny its copper or shiny brown color.
- Penny Changes to Silver Color
- Zn2+
- Zn2+
- Zn2+
- Zn2+
- Zn2+
- Zn2+
- The liquid solution contained Zinc powder (Zn2+), which made the gray solid in the beaker, and Potassium hydroxide (K+ and OH-). The solution was heated on a hot plate.
- Change in Composition of Silver Penny
- Zinc
- Zinc
- Zinc
- Zinc
- Zinc
- Zinc
- The penny was placed in the liquid solution being heated by the hot plate. The atoms also heated up and began to move around the beaker more frequently.
- Flame Forms Alloy Turning Penny Gold
- K+
- The Zinc atoms reacted with the Copper atoms of the penny causing them to stick together. The Zinc on the outside of the penny gave it its silver color.
- Zn2+
- Zn2+
- Zn2+
- Zn2+
- Copper
- Zn2+
- Zinc
- Zn2+
- Zn2+
- Zn2+
- Zn2+
- Zn2+
- Zn2+
- Zn2+
- The Zinc atoms from the core of the penny moved to the outside of the penny forming a layer of Zinc atoms surrounding the Copper atoms.
- Zinc
- Zinc
- Zinc
- Cu
- Cu
- Zinc
- Cu
- Cu
- Zinc
- Cu
- Cu
- Zinc
- Cu
- Cu
- Zinc
- Cu
- Cu
- Zinc
- Zinc
- Zinc
- When the penny was placed in the flame, the Copper and Zinc atoms mixed together to form an alloy. The alloy formed was brass which provided the gold color for the penny.
- Zn
- Cu
- Zn
- Cu
- Cu
- Zn
- Cu
- Zn
- Zn
- Zn
- Cu
- Cu
- Zn
- Cu
- Cu
- Zn
- Zn
- Cu
- Zn
- Cu
- Zn
- Cu
- Cu
- Zn
Over 30 Million Storyboards Created

