Chemistry
Storyboard Text
- H
- F
- F
- H
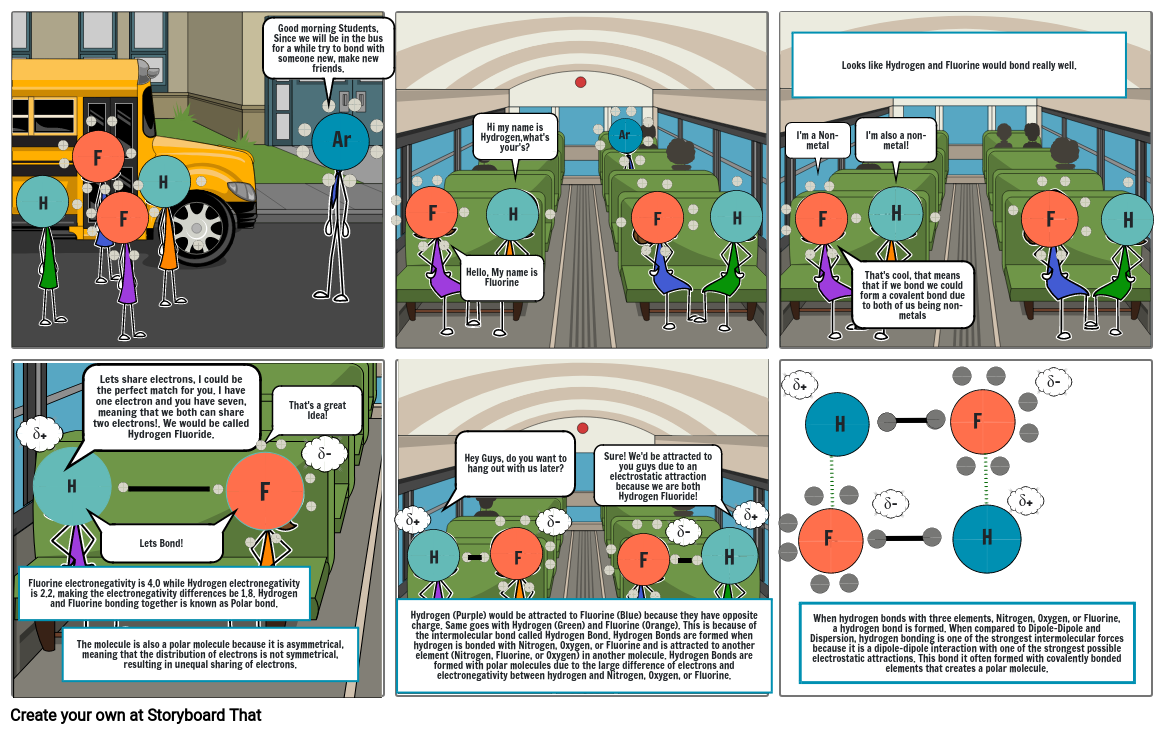
- Good morning Students, Since we will be in the bus for a while try to bond with someone new, make new friends.
- Ar
- F
- Hello, My name is Fluorine
- Hi my name is Hydrogen,what's your's?
- H
- Ar
- F
- H
- I'm a Non-metal
- Looks like Hydrogen and Fluorine would bond really well.
- F
- That's cool, that means that if we bond we could form a covalent bond due to both of us being non-metals
- H
- I'm also a non-metal!
- F
- H
- δ+
- Fluorine electronegativity is 4.0 while Hydrogen electronegativity is 2.2, making the electronegativity differences be 1.8. Hydrogen and Fluorine bonding together is known as Polar bond.
- H
- The molecule is also a polar molecule because it is asymmetrical, meaning that the distribution of electrons is not symmetrical, resulting in unequal sharing of electrons.
- Lets share electrons, I could be the perfect match for you. I have one electron and you have seven, meaning that we both can share two electrons!. We would be called Hydrogen Fluoride.
- Lets Bond!
- F
- δ-
- That's a great Idea!
- δ+
- Hydrogen (Purple) would be attracted to Fluorine (Blue) because they have opposite charge. Same goes with Hydrogen (Green) and Fluorine (Orange). This is because of the intermolecular bond called Hydrogen Bond. Hydrogen Bonds are formed when hydrogen is bonded with Nitrogen, Oxygen, or Fluorine and is attracted to another element (Nitrogen, Fluorine, or Oxygen) in another molecule. Hydrogen Bonds are formed with polar molecules due to the large difference of electrons and electronegativity between hydrogen and Nitrogen, Oxygen, or Fluorine.
- H
- F
- δ-
- Hey Guys, do you want to hang out with us later?
- F
- δ-
- H
- δ+
- Sure! We'd be attracted to you guys due to an electrostatic attraction because we are both Hydrogen Fluoride!
- δ+
- When hydrogen bonds with three elements, Nitrogen, Oxygen, or Fluorine, a hydrogen bond is formed. When compared to Dipole-Dipole and Dispersion, hydrogen bonding is one of the strongest intermolecular forces because it is a dipole-dipole interaction with one of the strongest possible electrostatic attractions. This bond it often formed with covalently bonded elements that creates a polar molecule.
- F
- H
- H
- δ-
- F
- H
- δ+
- δ-
Over 30 Million Storyboards Created

