chem ma
Storyboard Text
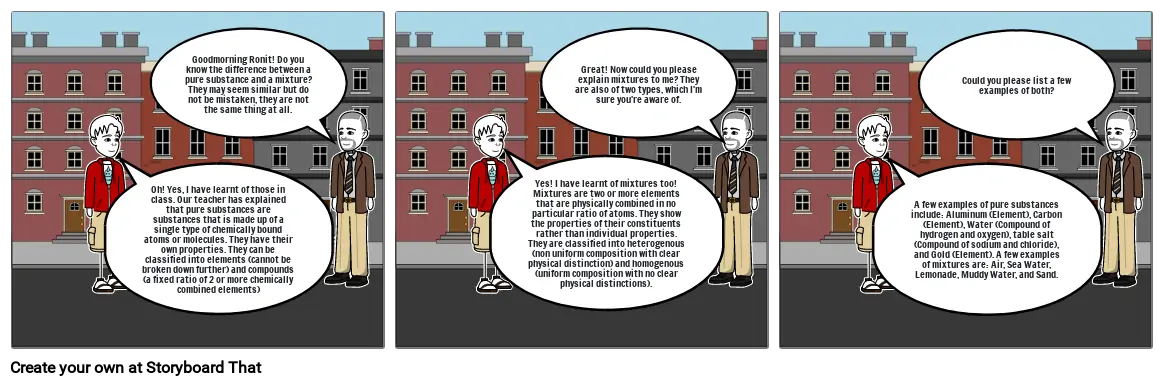
- Oh! Yes, I have learnt of those in class. Our teacher has explained that pure substances are substances that is made up of a single type of chemically bound atoms or molecules. They have their own properties. They can be classified into elements (cannot be broken down further) and compounds (a fixed ratio of 2 or more chemically combined elements)
- Goodmorning Ronit! Do you know the difference between a pure substance and a mixture? They may seem similar but do not be mistaken, they are not the same thing at all.
- Yes! I have learnt of mixtures too! Mixtures are two or more elements that are physically combined in no particular ratio of atoms. They show the properties of their constituents rather than individual properties. They are classified into heterogenous (non uniform composition with clear physical distinction) and homogenous (uniform composition with no clear physical distinctions).
- Great! Now could you please explain mixtures to me? They are also of two types, which I'm sure you're aware of.
- A few examples of pure substances include: Aluminum (Element), Carbon (Element), Water (Compound of hydrogen and oxygen), table salt (Compound of sodium and chloride), and Gold (Element). A few examples of mixtures are: Air, Sea Water, Lemonade, Muddy Water, and Sand.
- Could you please list a few examples of both?
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!
