Atomic Structure
Storyboard Text
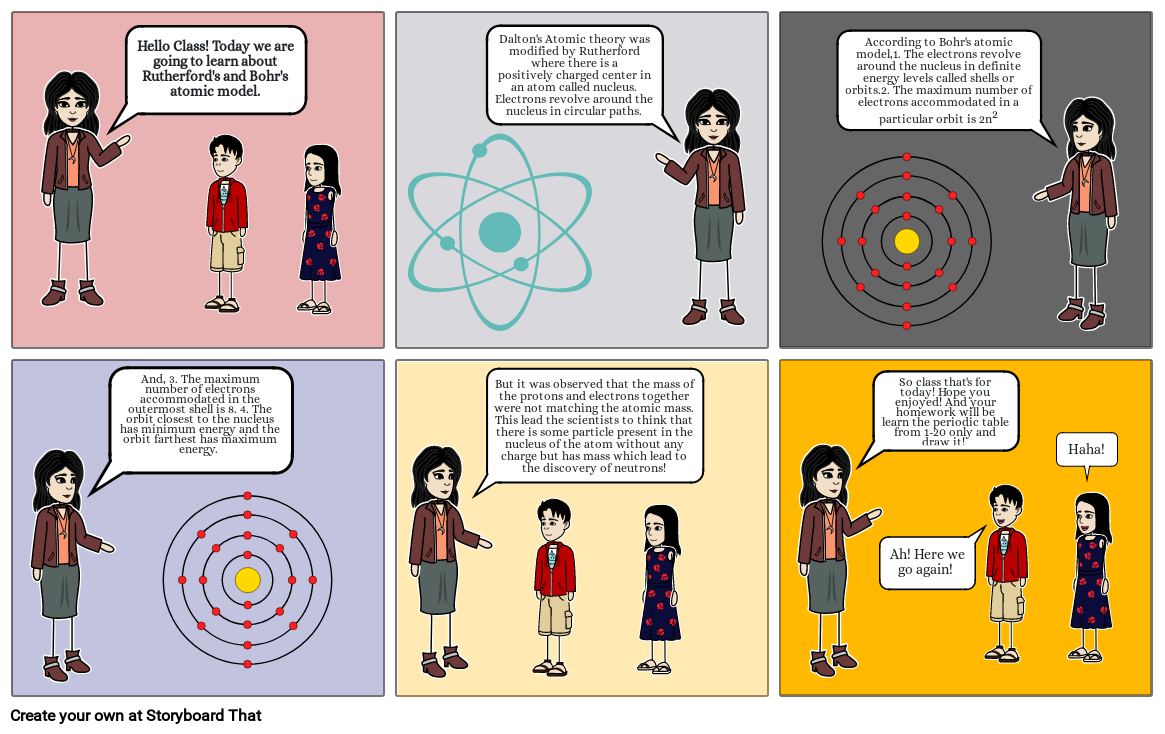
- Hello Class! Today we are going to learn about Rutherford's and Bohr's atomic model.
- Dalton's Atomic theory was modified by Rutherford where there is a positively charged center in an atom called nucleus. Electrons revolve around the nucleus in circular paths.
- According to Bohr's atomic model,1. The electrons revolve around the nucleus in definite energy levels called shells or orbits.2. The maximum number of electrons accommodated in a particular orbit is 2n2
- And, 3. The maximum number of electrons accommodated in the outermost shell is 8. 4. The orbit closest to the nucleus has minimum energy and the orbit farthest has maximum energy. 
- But it was observed that the mass of the protons and electrons together were not matching the atomic mass. This lead the scientists to think that there is some particle present in the nucleus of the atom without any charge but has mass which lead to the discovery of neutrons!
- So class that's for today! Hope you enjoyed! And your homework will be learn the periodic table from 1-20 only and draw it! 
- Ah! Here we go again! 
- Haha!
Over 30 Million Storyboards Created

