The Lemonade Stand 2
Storyboard Text
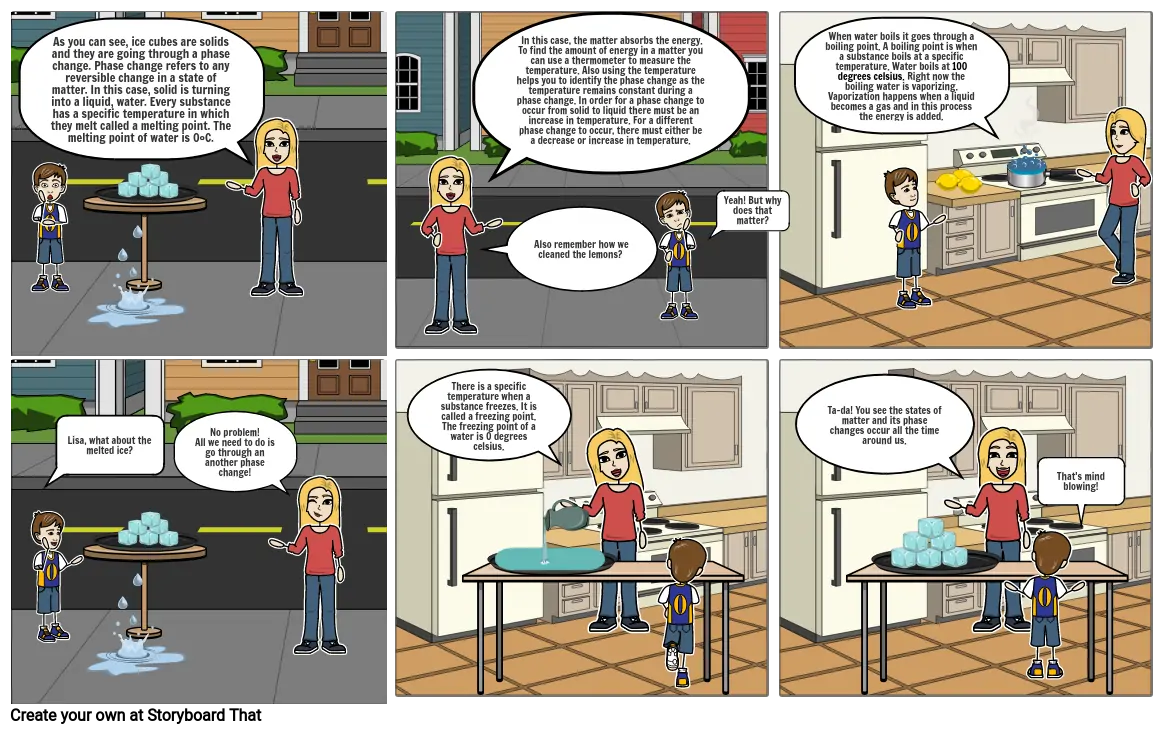
- As you can see, ice cubes are solids and they are going through a phase change. Phase change refers to any reversible change in a state of matter. In this case, solid is turning into a liquid, water. Every substance has a specific temperature in which they melt called a melting point. The melting point of water is 0°C.
- In this case, the matter absorbs the energy. To find the amount of energy in a matter you can use a thermometer to measure the temperature. Also using the temperature helps you to identify the phase change as the temperature remains constant during a phase change. In order for a phase change to occur from solid to liquid there must be an increase in temperature. For a different phase change to occur, there must either be a decrease or increase in temperature.
- Also remember how we cleaned the lemons?
- Yeah! But why does that matter?
- When water boils it goes through a boiling point. A boiling point is when a substance boils at a specific temperature. Water boils at 100 degrees celsius. Right now the boiling water is vaporizing. Vaporization happens when a liquid becomes a gas and in this process the energy is added.
- Lisa, what about the melted ice?
- No problem! All we need to do is go through an another phase change!
- There is a specific temperature when a substance freezes. It is called a freezing point. The freezing point of a water is 0 degrees celsius.
- Ta-da! You see the states of matter and its phase changes occur all the time around us.
- That's mind blowing!
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!
