States of matter
Storyboard Text
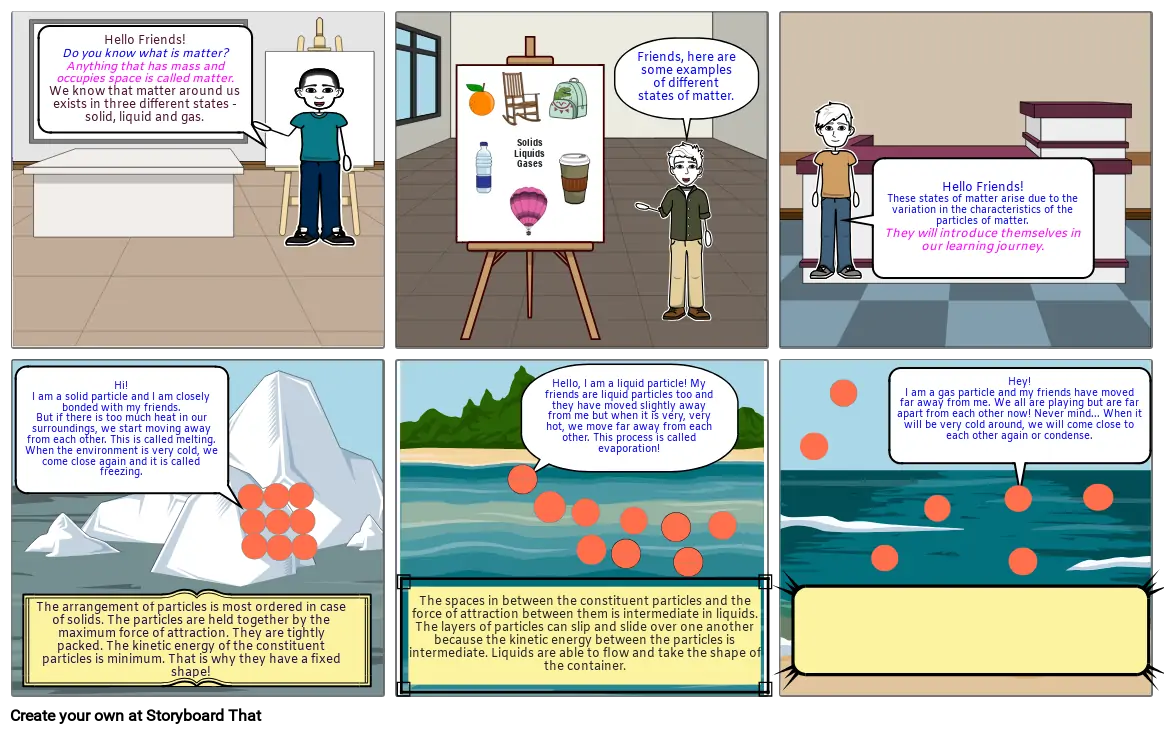
- Slide: 1
- Hello Friends!Do you know what is matter?Anything that has mass and occupies space is called matter.We know that matter around us exists in three different states - solid, liquid and gas.
- Slide: 2
- SolidsLiquidsGases
- Friends, here are some examples of different states of matter.
- Slide: 3
- Hello Friends!These states of matter arise due to the variation in the characteristics of the particles of matter.They will introduce themselves in our learning journey.
- Slide: 4
- Hi!I am a solid particle and I am closely bonded with my friends.But if there is too much heat in our surroundings, we start moving away from each other. This is called melting.When the environment is very cold, we come close again and it is called freezing.
- The arrangement of particles is most ordered in case of solids. The particles are held together by the maximum force of attraction. They are tightly packed. The kinetic energy of the constituent particles is minimum. That is why they have a fixed shape!
- Slide: 5
- Hello, I am a liquid particle! My friends are liquid particles too and they have moved slightly away from me but when it is very, very hot, we move far away from each other. This process is called evaporation!
- The spaces in between the constituent particles and the force of attraction between them is intermediate in liquids.The layers of particles can slip and slide over one another because the kinetic energy between the particles is intermediate. Liquids are able to flow and take the shape of the container.
- Slide: 6
- Hey!I am a gas particle and my friends have moved far away from me. We all are playing but are far apart from each other now! Never mind... When it will be very cold around, we will come close to each other again or condense.
- The force of attraction between the gas particles is minimum. The spaces in between the constituent particles and the kinetic energy is maximum in gases. There is no order in the arrangement of particles in gases and they just move about randomly.
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!
