BOYLES LAW
Storyboard Text
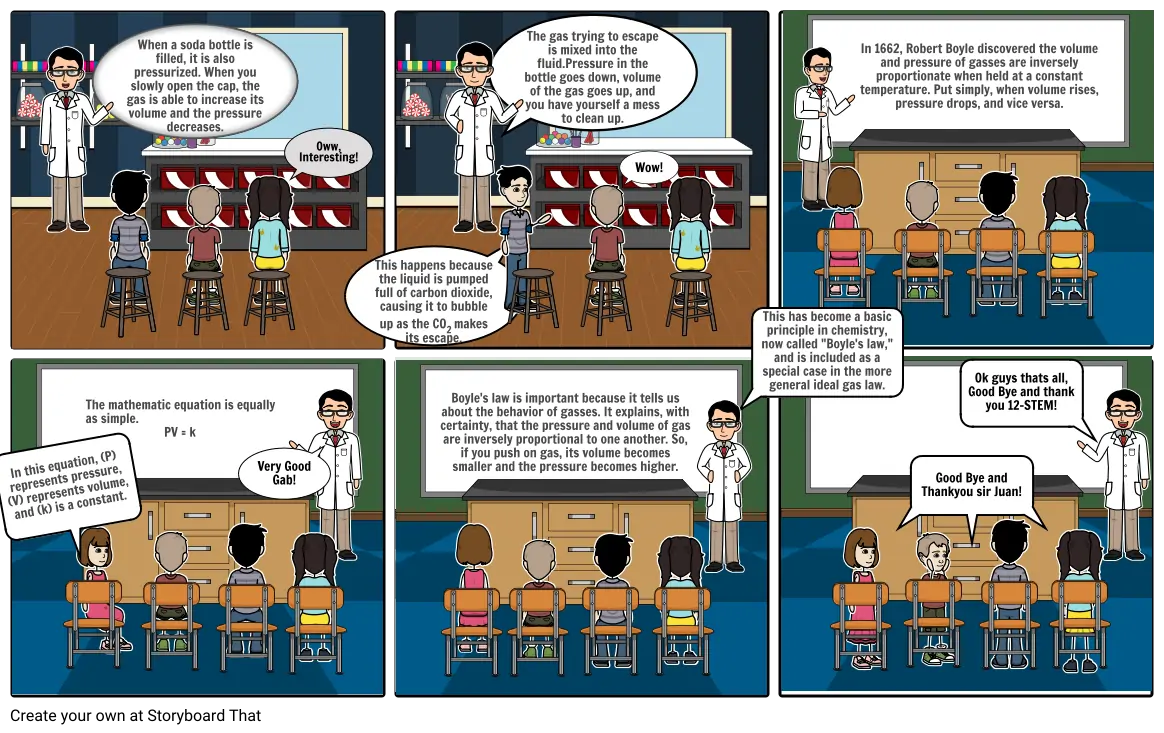
- When a soda bottle is filled, it is also pressurized. When you slowly open the cap, the gas is able to increase its volume and the pressure decreases.
- Oww, Interesting!
- This happens because the liquid is pumped full of carbon dioxide, causing it to bubble up as the CO2 makes its escape.
- The gas trying to escape is mixed into the fluid.Pressure in the bottle goes down, volume of the gas goes up, and you have yourself a mess to clean up.
- Wow!
- This has become a basic principle in chemistry, now called "Boyle's law," and is included as a special case in the more general ideal gas law.
- In 1662, Robert Boyle discovered the volume and pressure of gasses are inversely proportionate when held at a constant temperature. Put simply, when volume rises, pressure drops, and vice versa.
- In this equation, (P) represents pressure, (V) represents volume, and (k) is a constant.
- The mathematic equation is equally as simple. PV = k
- Very Good Gab!
- Boyle's law is important because it tells us about the behavior of gasses. It explains, with certainty, that the pressure and volume of gas are inversely proportional to one another. So, if you push on gas, its volume becomes smaller and the pressure becomes higher.
- Good Bye and Thankyou sir Juan!
- Ok guys thats all, Good Bye and thank you 12-STEM!
Over 30 Million Storyboards Created

