Unknown Story
Storyboard Text
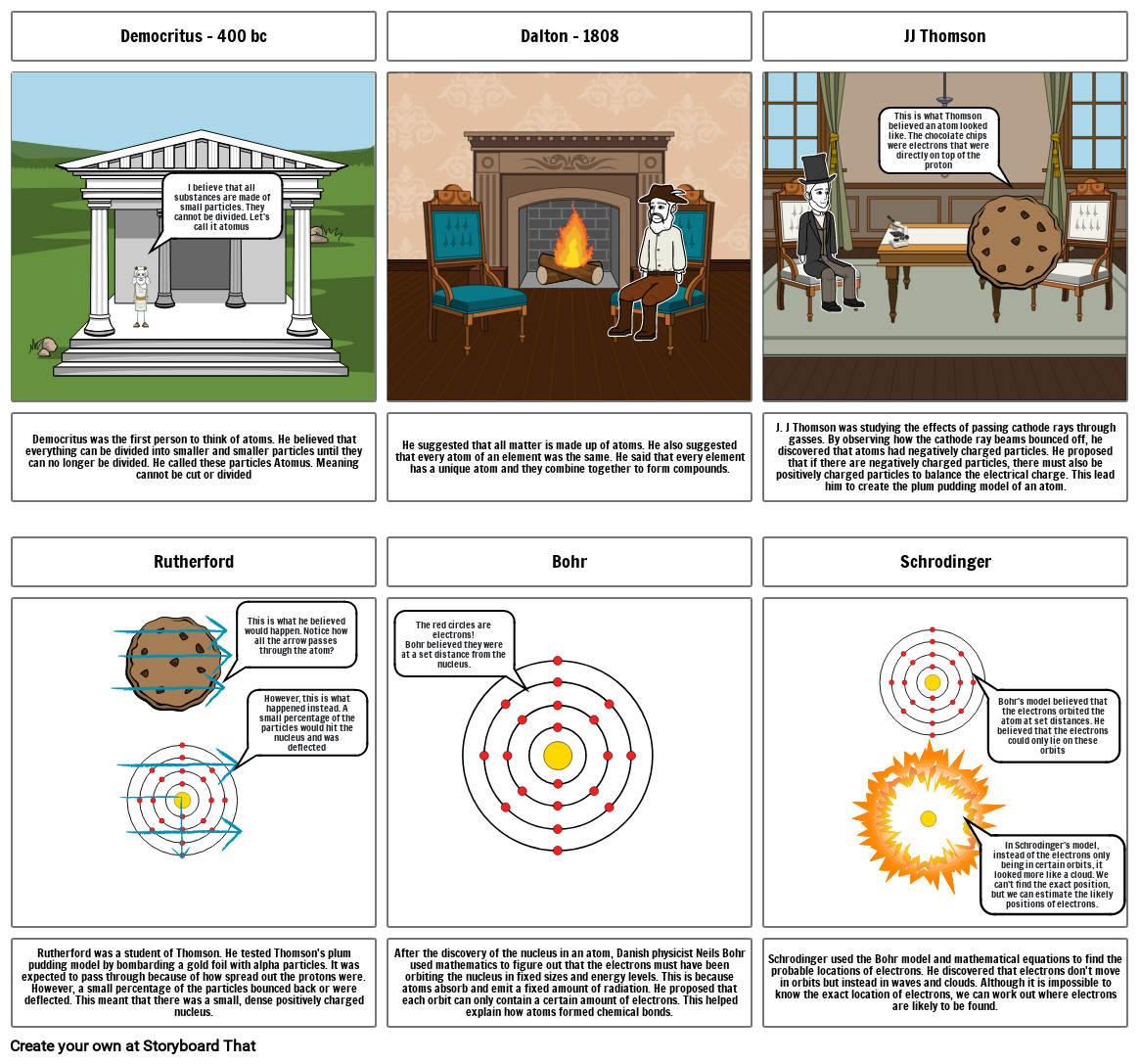
- Democritus - 400 bc
- I believe that all substances are made of small particles. They cannot be divided. Let's call it atomus
- Dalton - 1808
- JJ Thomson
- This is what Thomson believed an atom looked like. The chocolate chips were electrons that were directly on top of the proton
- Democritus was the first person to think of atoms. He believed that everything can be divided into smaller and smaller particles until they can no longer be divided. He called these particles Atomus. Meaning cannot be cut or divided
- Rutherford
- This is what he believed would happen. Notice how all the arrow passes through the atom?
- He suggested that all matter is made up of atoms. He also suggested that every atom of an element was the same. He said that every element has a unique atom and they combine together to form compounds.
- Bohr
- The red circles are electrons!Bohr believed they were at a set distance from the nucleus.
- J. J Thomson was studying the effects of passing cathode rays through gasses. By observing how the cathode ray beams bounced off, he discovered that atoms had negatively charged particles. He proposed that if there are negatively charged particles, there must also be positively charged particles to balance the electrical charge. This lead him to create the plum pudding model of an atom.
- Schrodinger
- Bohr's model believed that the electrons orbited the atom at set distances. He believed that the electrons could only lie on these orbits
- Rutherford was a student of Thomson. He tested Thomson's plum pudding model by bombarding a gold foil with alpha particles. It was expected to pass through because of how spread out the protons were. However, a small percentage of the particles bounced back or were deflected. This meant that there was a small, dense positively charged nucleus.
- However, this is what happened instead. A small percentage of the particles would hit the nucleus and was deflected
- After the discovery of the nucleus in an atom, Danish physicist Neils Bohr used mathematics to figure out that the electrons must have been orbiting the nucleus in fixed sizes and energy levels. This is because atoms absorb and emit a fixed amount of radiation. He proposed that each orbit can only contain a certain amount of electrons. This helped explain how atoms formed chemical bonds.
- Schrodinger used the Bohr model and mathematical equations to find the probable locations of electrons. He discovered that electrons don't move in orbits but instead in waves and clouds. Although it is impossible to know the exact location of electrons, we can work out where electrons are likely to be found.
- In Schrodinger's model, instead of the electrons only being in certain orbits, it looked more like a cloud. We can't find the exact position, but we can estimate the likely positions of electrons.
Over 30 Million Storyboards Created

