Sessions Why, why, why...
Storyboard Text
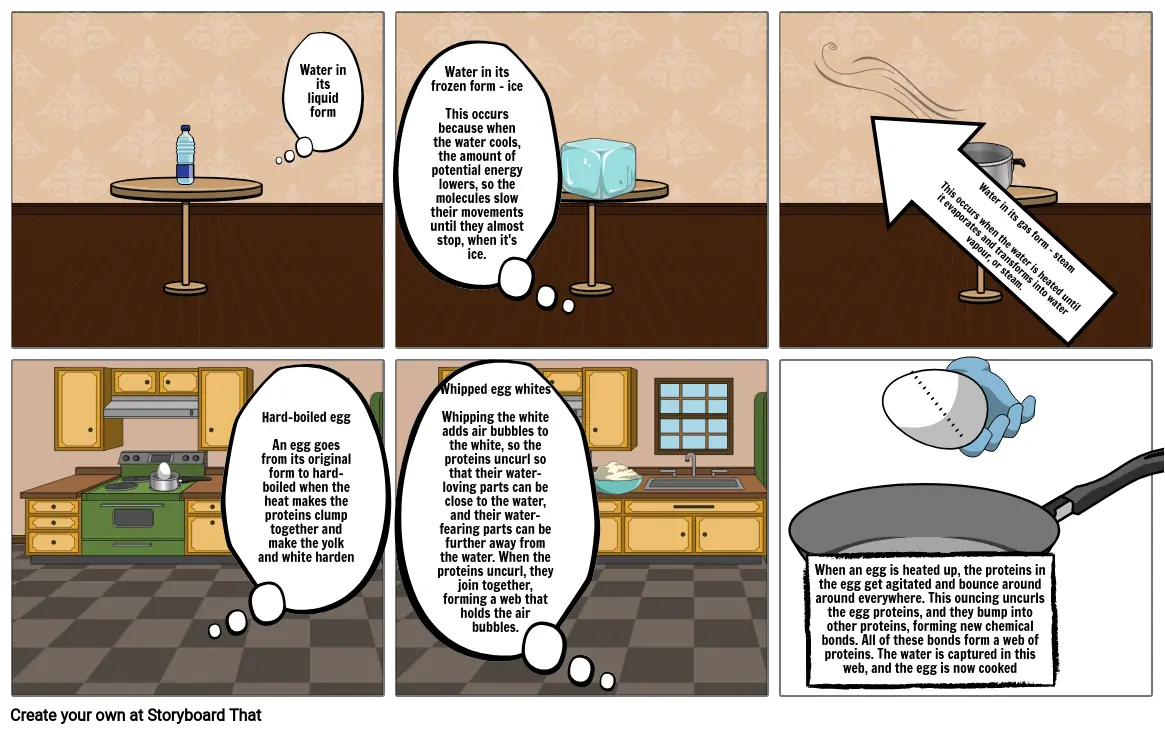
- Water in its liquid form
- Water in its frozen form - iceThis occurs because when the water cools, the amount of potential energy lowers, so the molecules slow their movements until they almost stop, when it's ice.
- Water in its gas form - steamThis occurs when the water is heated until it evaporates and transforms into water vapour, or steam.
- Hard-boiled eggAn egg goes from its original form to hard-boiled when the heat makes the proteins clump together and make the yolk and white harden
- Whipped egg whitesWhipping the white adds air bubbles to the white, so the proteins uncurl so that their water-loving parts can be close to the water, and their water-fearing parts can be further away from the water. When the proteins uncurl, they join together, forming a web that holds the air bubbles.
- When an egg is heated up, the proteins in the egg get agitated and bounce around around everywhere. This ouncing uncurls the egg proteins, and they bump into other proteins, forming new chemical bonds. All of these bonds form a web of proteins. The water is captured in this web, and the egg is now cooked
Over 30 Million Storyboards Created

