History of the Atomic Theory
Storyboard Description
2020
Storyboard Text
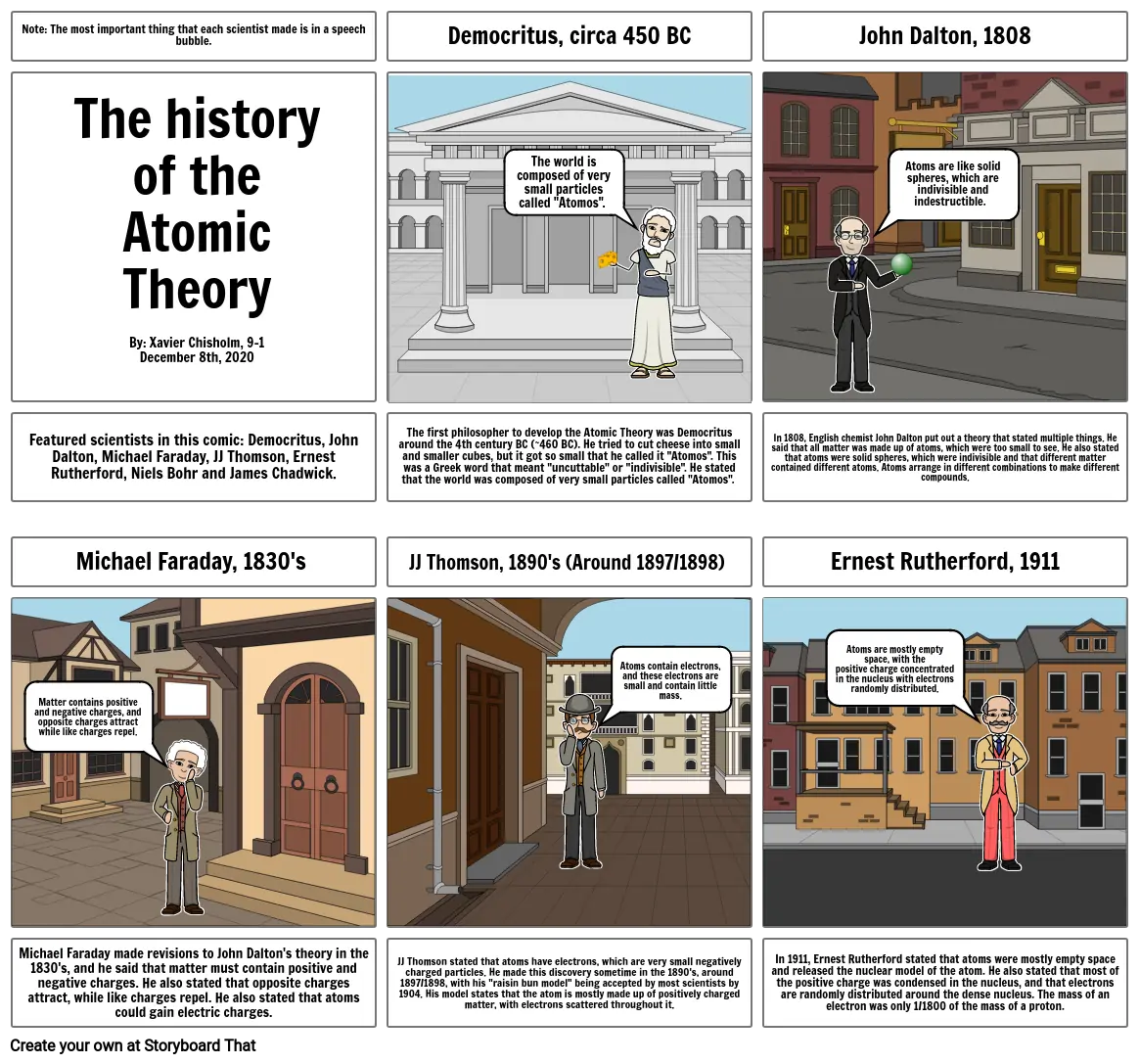
- Note: The most important thing that each scientist made is in a speech bubble.
- The history of the Atomic TheoryBy: Xavier Chisholm, 9-1December 8th, 2020
- Democritus, circa 450 BC
- The world is composed of very small particles called "Atomos".
- John Dalton, 1808
- Atoms are like solid spheres, which are indivisible and indestructible.
- Featured scientists in this comic: Democritus, John Dalton, Michael Faraday, JJ Thomson, Ernest Rutherford, Niels Bohr and James Chadwick.
- Michael Faraday, 1830's
- Matter contains positive and negative charges, and opposite charges attract while like charges repel.
- The first philosopher to develop the Atomic Theory was Democritus around the 4th century BC (~460 BC). He tried to cut cheese into small and smaller cubes, but it got so small that he called it "Atomos". This was a Greek word that meant "uncuttable" or "indivisible". He stated that the world was composed of very small particles called "Atomos".
- JJ Thomson, 1890's (Around 1897/1898)
- Atoms contain electrons, and these electrons are small and contain little mass.
- In 1808, English chemist John Dalton put out a theory that stated multiple things. He said that all matter was made up of atoms, which were too small to see. He also stated that atoms were solid spheres, which were indivisible and that different matter contained different atoms. Atoms arrange in different combinations to make different compounds.
- Ernest Rutherford, 1911
- Atoms are mostly empty space, with the positive charge concentrated in the nucleus with electrons randomly distributed.
- Michael Faraday made revisions to John Dalton's theory in the 1830's, and he said that matter must contain positive and negative charges. He also stated that opposite charges attract, while like charges repel. He also stated that atoms could gain electric charges.
- JJ Thomson stated that atoms have electrons, which are very small negatively charged particles. He made this discovery sometime in the 1890's, around 1897/1898, with his "raisin bun model" being accepted by most scientists by 1904. His model states that the atom is mostly made up of positively charged matter, with electrons scattered throughout it.
- In 1911, Ernest Rutherford stated that atoms were mostly empty space and released the nuclear model of the atom. He also stated that most of the positive charge was condensed in the nucleus, and that electrons are randomly distributed around the dense nucleus. The mass of an electron was only 1/1800 of the mass of a proton.
Over 30 Million Storyboards Created

