CHEMICAL EQUATION STRIP
Storyboard Text
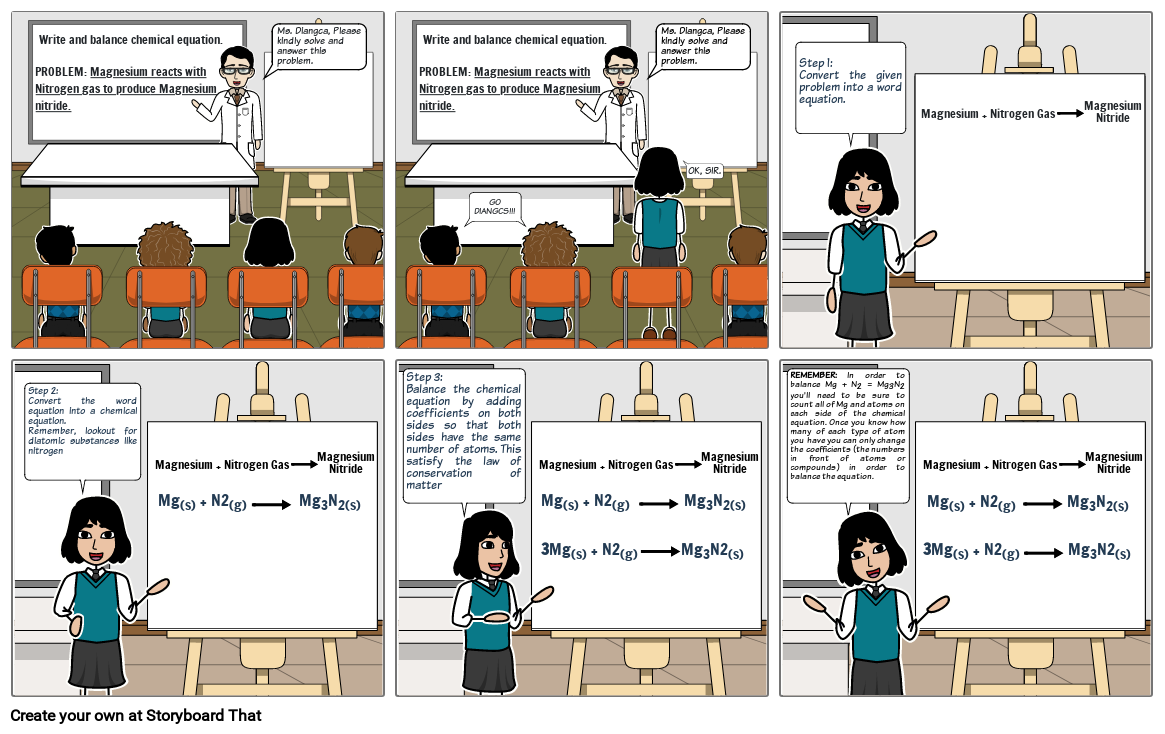
- Write and balance chemical equation.PROBLEM: Magnesium reacts with Nitrogen gas to produce Magnesium nitride.
- Ms. Diangca, Please kindly solve and answer this problem.
- Write and balance chemical equation.PROBLEM: Magnesium reacts with Nitrogen gas to produce Magnesium nitride.
- GO DIANGCS!!!
- Ms. Diangca, Please kindly solve and answer this problem.
- OK, SIR.
- Step 1: Convert the given problem into a word equation.
- Magnesium + Nitrogen Gas
- Magnesium Nitride
- Step 2:Convert the word equation into a chemical equation. Remember, lookout for diatomic substances like nitrogen
- Mg(s) + N2(g) DJASW Mg3N2(s)
- Magnesium + Nitrogen Gas
- Magnesium Nitride
- Step 3: Balance the chemical equation by adding coefficients on both sides so that both sides have the same number of atoms. This satisfy the law of conservation of matter
- Mg(s) + N2(g) HKASHD Mg3N2(s)
- 3Mg(s) + N2(g) HSAH Mg3N2(s)
- Magnesium + Nitrogen Gas
- Magnesium Nitride
- REMEMBER: In order to balance Mg + N2 = Mg3N2 you'll need to be sure to count all of Mg and atoms on each side of the chemical equation. Once you know how many of each type of atom you have you can only change the coefficients (the numbers in front of atoms or compounds) in order to balance the equation.
- Mg(s) + N2(g) DHKJAS Mg3N2(s)
- 3Mg(s) + N2(g) JADHJH Mg3N2(s)
- Magnesium + Nitrogen Gas
- Magnesium Nitride
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!

