Unknown Story
Storyboard Text
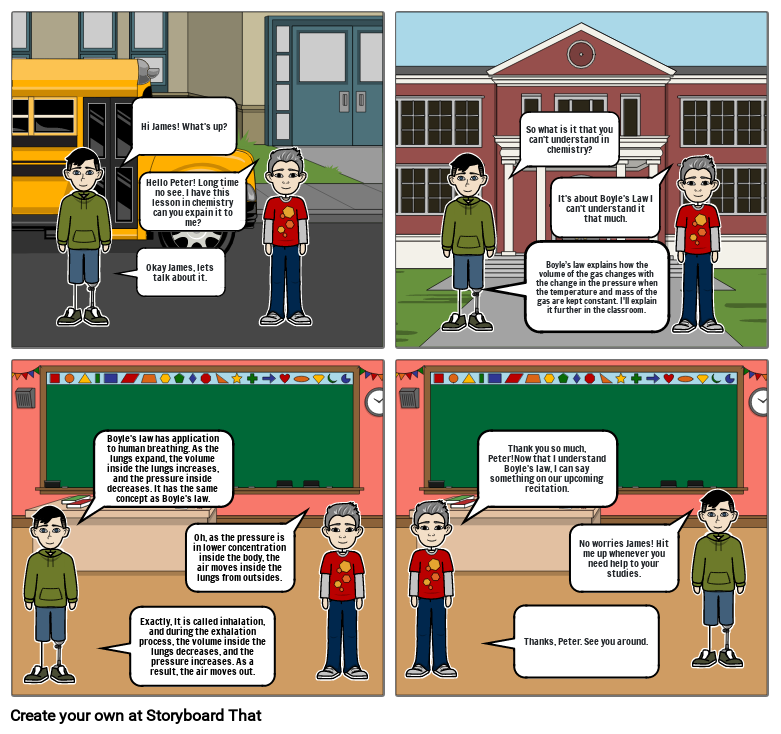
- Okay James, lets talk about it.
- Hi James! What's up?
- Hello Peter! Long time no see. I have this lesson in chemistry can you expain it to me?
- Boyle’s law explains how the volume of the gas changes with the change in the pressure when the temperature and mass of the gas are kept constant. I'll explain it further in the classroom.
- So what is it that you can't understand in chemistry?
- It's about Boyle's Law I can't understand it that much.
- Boyle's law has application to human breathing. As the lungs expand, the volume inside the lungs increases, and the pressure inside decreases. It has the same concept as Boyle's law.
- Exactly, It is called inhalation, and during the exhalation process, the volume inside the lungs decreases, and the pressure increases. As a result, the air moves out.
- Oh, as the pressure is in lower concentration inside the body, the air moves inside the lungs from outsides.
- Thank you so much, Peter!Now that I understand Boyle's law, I can say something on our upcoming recitation.
- Thanks, Peter. See you around.
- No worries James! Hit me up whenever you need help to your studies.
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!

