Separation of Substances Comic Strip (2)
Storyboard Text
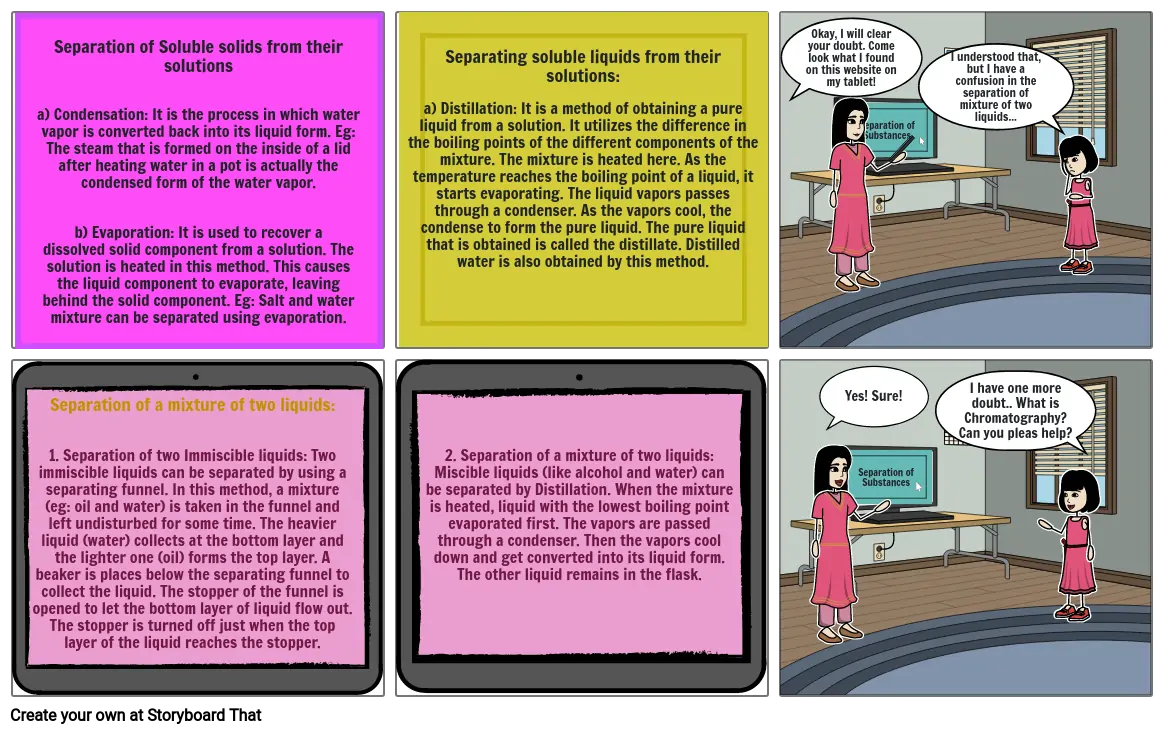
- Separation of Soluble solids from their solutionsa) Condensation: It is the process in which water vapor is converted back into its liquid form. Eg: The steam that is formed on the inside of a lid after heating water in a pot is actually the condensed form of the water vapor.b) Evaporation: It is used to recover a dissolved solid component from a solution. The solution is heated in this method. This causes the liquid component to evaporate, leaving behind the solid component. Eg: Salt and water mixture can be separated using evaporation.
- Separating soluble liquids from their solutions:a) Distillation: It is a method of obtaining a pure liquid from a solution. It utilizes the difference in the boiling points of the different components of the mixture. The mixture is heated here. As the temperature reaches the boiling point of a liquid, it starts evaporating. The liquid vapors passes through a condenser. As the vapors cool, the condense to form the pure liquid. The pure liquid that is obtained is called the distillate. Distilled water is also obtained by this method.
- Okay, I will clear your doubt. Come look what I found on this website on my tablet!
- Separation of Substances
- I understood that, but I have a confusion in the separation of mixture of two liquids...
- Separation of a mixture of two liquids:1. Separation of two Immiscible liquids: Two immiscible liquids can be separated by using a separating funnel. In this method, a mixture (eg: oil and water) is taken in the funnel and left undisturbed for some time. The heavier liquid (water) collects at the bottom layer and the lighter one (oil) forms the top layer. A beaker is places below the separating funnel to collect the liquid. The stopper of the funnel is opened to let the bottom layer of liquid flow out. The stopper is turned off just when the top layer of the liquid reaches the stopper.
- 2. Separation of a mixture of two liquids: Miscible liquids (like alcohol and water) can be separated by Distillation. When the mixture is heated, liquid with the lowest boiling point evaporated first. The vapors are passed through a condenser. Then the vapors cool down and get converted into its liquid form. The other liquid remains in the flask.
- Yes! Sure!
- Separation of Substances
- I have one more doubt.. What is Chromatography? Can you pleas help?
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!
