Untitled Storyboard
Storyboard Text
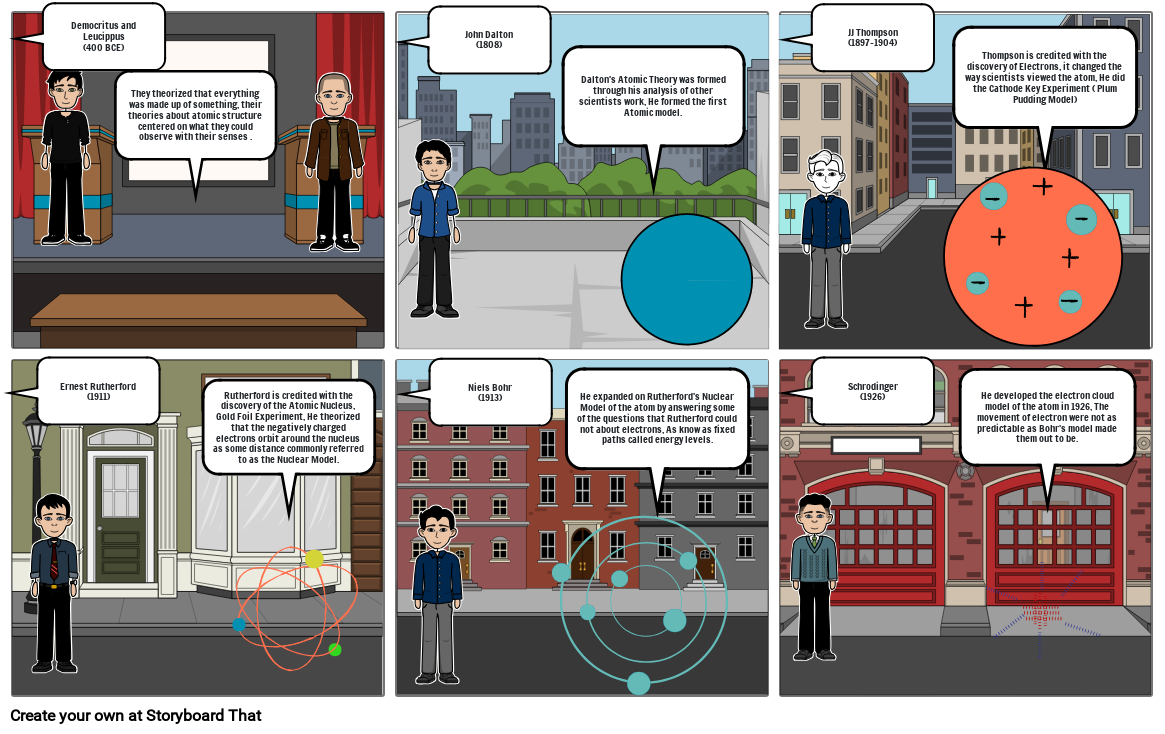
- Slide: 1
- Democritus and Leucippus(400 BCE)
- They theorized that everything was made up of something, their theories about atomic structure centered on what they could observe with their senses .
- Slide: 2
- John Dalton(1808)
- Dalton's Atomic Theory was formed through his analysis of other scientists work, He formed the first Atomic model.
- Slide: 3
- JJ Thompson(1897-1904)
- Thompson is credited with the discovery of Electrons, it changed the way scientists viewed the atom, He did the Cathode Key Experiment ( Plum Pudding Model)
- Slide: 4
- Ernest Rutherford(1911)
- Rutherford is credited with the discovery of the Atomic Nucleus, Gold Foil Experiment, He theorized that the negatively charged electrons orbit around the nucleus as some distance commonly referred to as the Nuclear Model.
- Slide: 5
- Niels Bohr (1913)
- He expanded on Rutherford's Nuclear Model of the atom by answering some of the questions that Rutherford could not about electrons, As know as fixed paths called energy levels.
- Slide: 6
- Schrodinger (1926)
- He developed the electron cloud model of the atom in 1926, The movement of electron were not as predictable as Bohr's model made them out to be.
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!
