Unknown Story
Storyboard Text
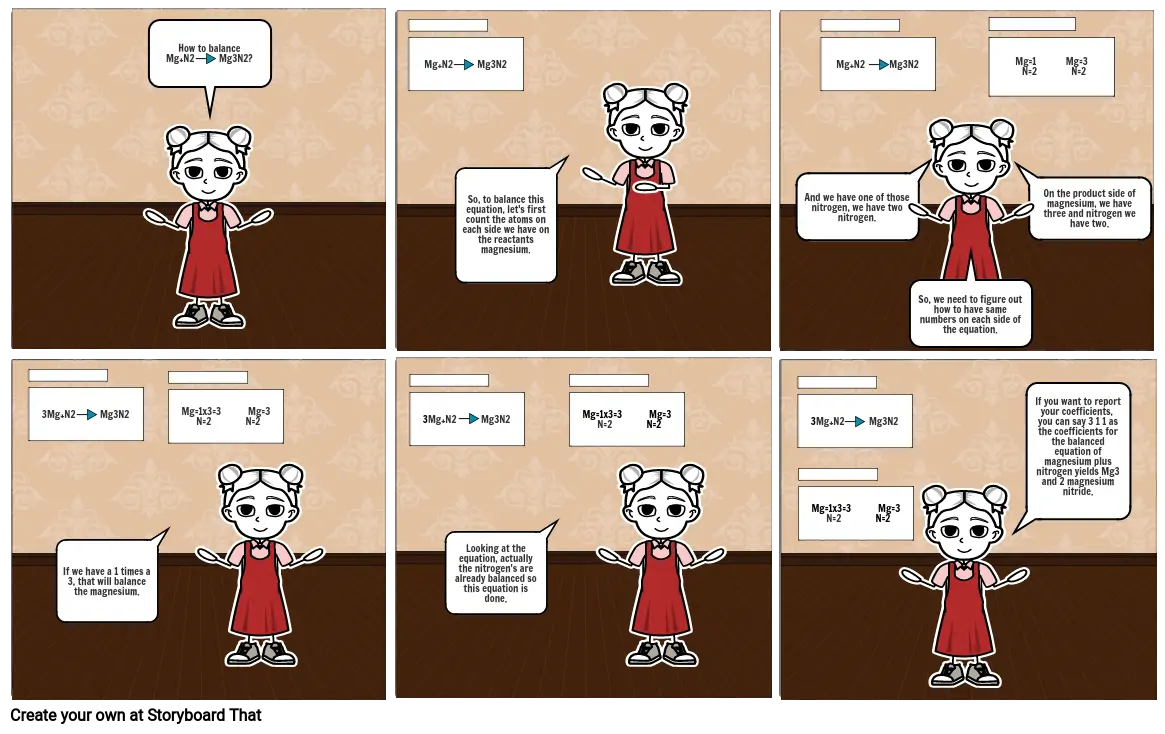
- How to balance Mg+N2 Mg3N2?
- Mg+N2 Mg3N2
- So, to balance this equation, let's first count the atoms on each side we have on the reactants magnesium.
- And we have one of those nitrogen, we have two nitrogen.
- Mg+N2 Mg3N2
- So, we need to figure out how to have same numbers on each side of the equation.
- Mg=1 Mg=3 N=2 N=2
- On the product side of magnesium, we have three and nitrogen we have two.
- 3Mg+N2 Mg3N2
- If we have a 1 times a 3, that will balance the magnesium.
- Mg=1x3=3 Mg=3 N=2 N=2
- 3Mg+N2 Mg3N2
- Looking at the equation, actually the nitrogen's are already balanced so this equation is done.
- Mg=1x3=3 Mg=3 N=2 N=2
- 3Mg+N2 Mg3N2
- Mg=1x3=3 Mg=3 N=2 N=2
- If you want to report your coefficients, you can say 3 1 1 as the coefficients for the balanced equation of magnesium plus nitrogen yields Mg3 and 2 magnesium nitride.
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!


