Cian atomic theory
Storyboard Text
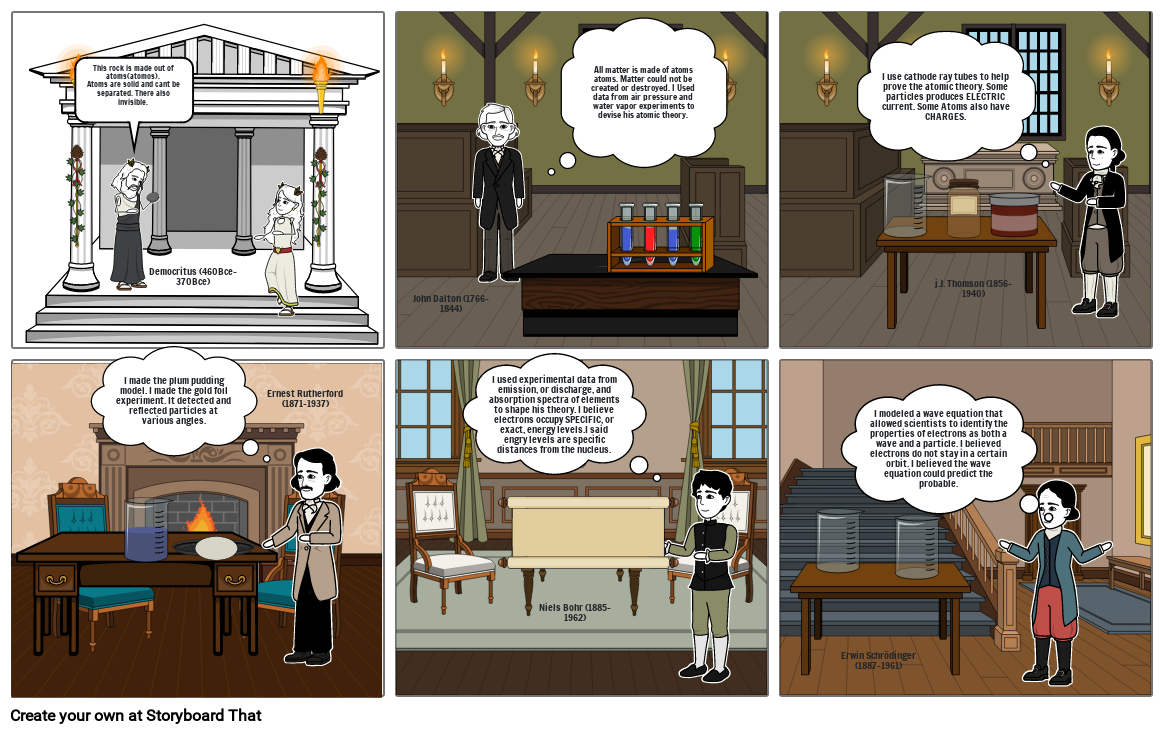
- This rock is made out of atoms(atomos).Atoms are solid and cant be separated. There also invisible.
- I made the plum pudding model. I made the gold foil experiment. It detected and reflected particles at various angles.
- Democritus (460Bce-370Bce)
- John Dalton (1766-1844)
- All matter is made of atoms atoms. Matter could not be created or destroyed. I Used data from air pressure and water vapor experiments to devise his atomic theory.
- I use cathode ray tubes to help prove the atomic theory. Some particles produces ELECTRIC current. Some Atoms also have CHARGES.
- j.J. Thomson (1856-1940)
- Ernest Rutherford (1871-1937)
- I used experimental data from emission, or discharge, and absorption spectra of elements to shape his theory. I believe electrons occupy SPECIFIC, or exact, energy levels.I said engry levels are specific distances from the nucleus.
- Niels Bohr (1885-1962)
- Erwin Schrödinger (1887-1961)
- I modeled a wave equation that allowed scientists to identify the properties of electrons as both a wave and a particle. I believed electrons do not stay in a certain orbit. I believed the wave equation could predict the probable.
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!


