Scientist
Storyboard Text
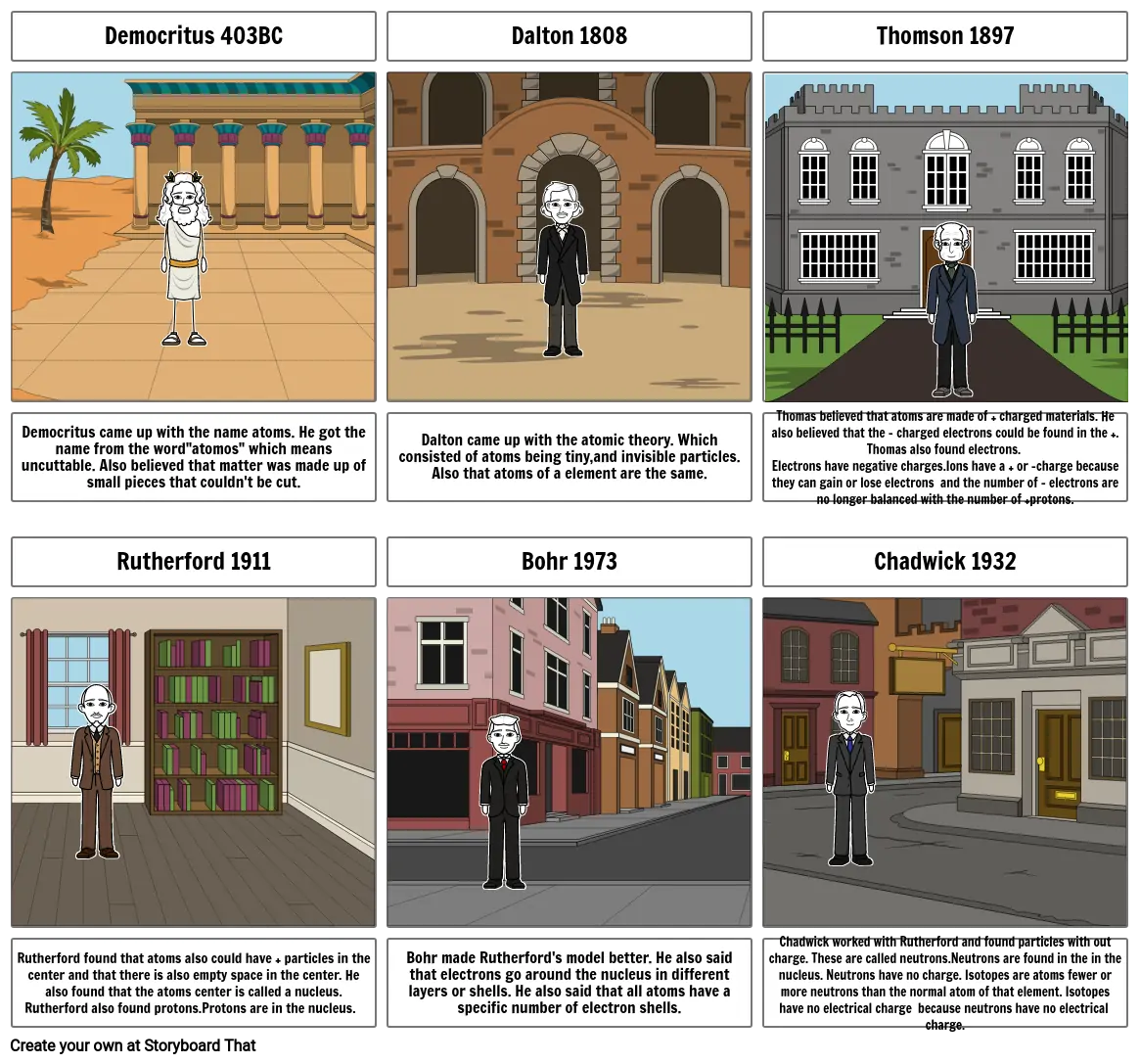
- Democritus 403BC
- Dalton 1808
- Thomson 1897
- Democritus came up with the name atoms. He got the name from the word"atomos" which means uncuttable. Also believed that matter was made up of small pieces that couldn't be cut.
- Rutherford 1911
- Dalton came up with the atomic theory. Which consisted of atoms being tiny,and invisible particles. Also that atoms of a element are the same.
- Bohr 1973
- Thomas believed that atoms are made of + charged materials. He also believed that the - charged electrons could be found in the +. Thomas also found electrons. Electrons have negative charges.Ions have a + or -charge because they can gain or lose electrons and the number of - electrons are no longer balanced with the number of +protons.
- Chadwick 1932
- Rutherford found that atoms also could have + particles in the center and that there is also empty space in the center. He also found that the atoms center is called a nucleus.Rutherford also found protons.Protons are in the nucleus.
- Bohr made Rutherford's model better. He also said that electrons go around the nucleus in different layers or shells. He also said that all atoms have a specific number of electron shells.
- Chadwick worked with Rutherford and found particles with out charge. These are called neutrons.Neutrons are found in the in the nucleus. Neutrons have no charge. Isotopes are atoms fewer or more neutrons than the normal atom of that element. Isotopes have no electrical charge because neutrons have no electrical charge.
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!


