Lilly F.- Atomic theory
Storyboard Text
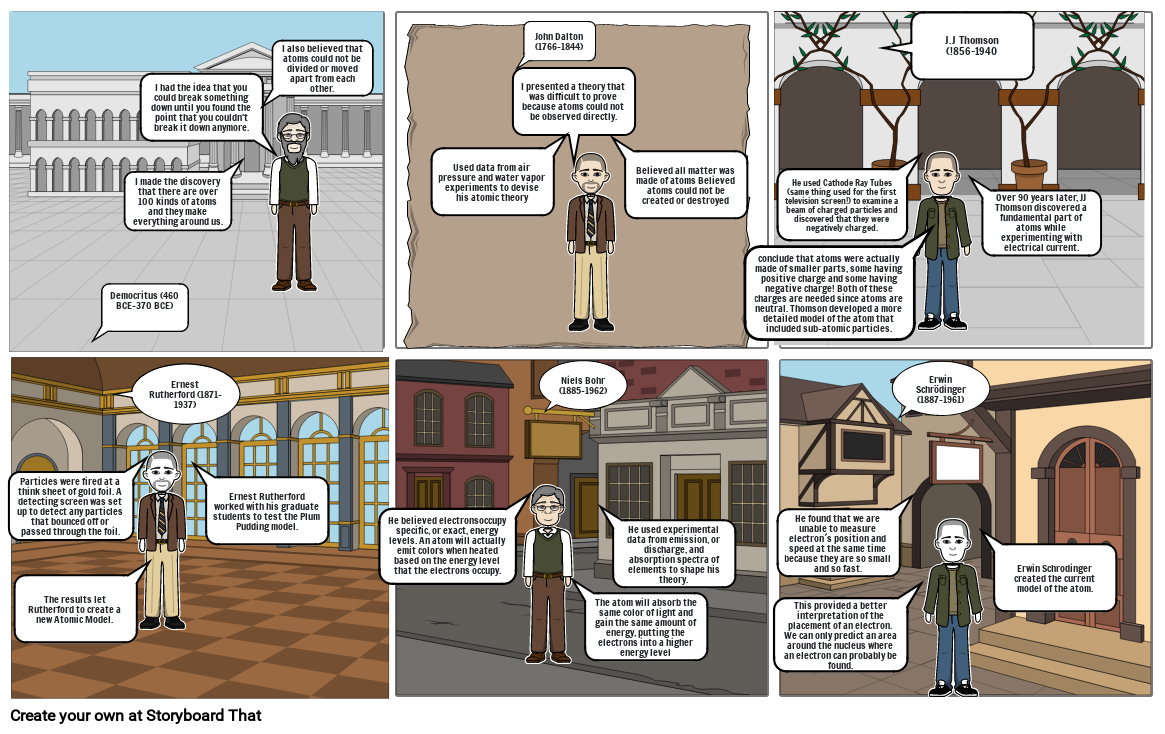
- Democritus (460 BCE-370 BCE)
- I made the discovery that there are over 100 kinds of atoms and they make everything around us.
- I had the idea that you could break something down until you found the point that you couldn't break it down anymore.
- I also believed that atoms could not be divided or moved apart from each other.
- Used data from air pressure and water vapor experiments to devise his atomic theory
- I presented a theory that was difficult to prove because atoms could not be observed directly.
- John Dalton (1766-1844)
- Believed all matter was made of atoms Believed atoms could not be created or destroyed
- conclude that atoms were actually made of smaller parts, some having positive charge and some having negative charge! Both of these charges are needed since atoms are neutral. Thomson developed a more detailed model of the atom that included sub-atomic particles.
- He used Cathode Ray Tubes (same thing used for the first television screen!) to examine a beam of charged particles and discovered that they were negatively charged.
- J.J Thomson(!856-1940
- Over 90 years later, JJ Thomson discovered a fundamental part of atoms while experimenting with electrical current.
- Particles were fired at a think sheet of gold foil. A detecting screen was set up to detect any particles that bounced off or passed through the foil.
- The results let Rutherford to create a new Atomic Model.
- Ernest Rutherford (1871-1937)
- Ernest Rutherford worked with his graduate students to test the Plum Pudding model.
- He believed electronsoccupy specific, or exact, energy levels. An atom will actually emit colors when heated based on the energy level that the electrons occupy.
- Niels Bohr (1885-1962)
- The atom will absorb the same color of light and gain the same amount of energy, putting the electrons into a higher energy level
- He used experimental data from emission, or discharge, and absorption spectra of elements to shape his theory.
- This provided a better interpretation of the placement of an electron. We can only predict an area around the nucleus where an electron can probably be found.
- He found that we are unable to measure electron´s position and speed at the same time because they are so small and so fast.
- Erwin Schrödinger (1887-1961)
- Erwin Schrodinger created the current model of the atom.
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!


