Early Atomic Theory
Storyboard Text
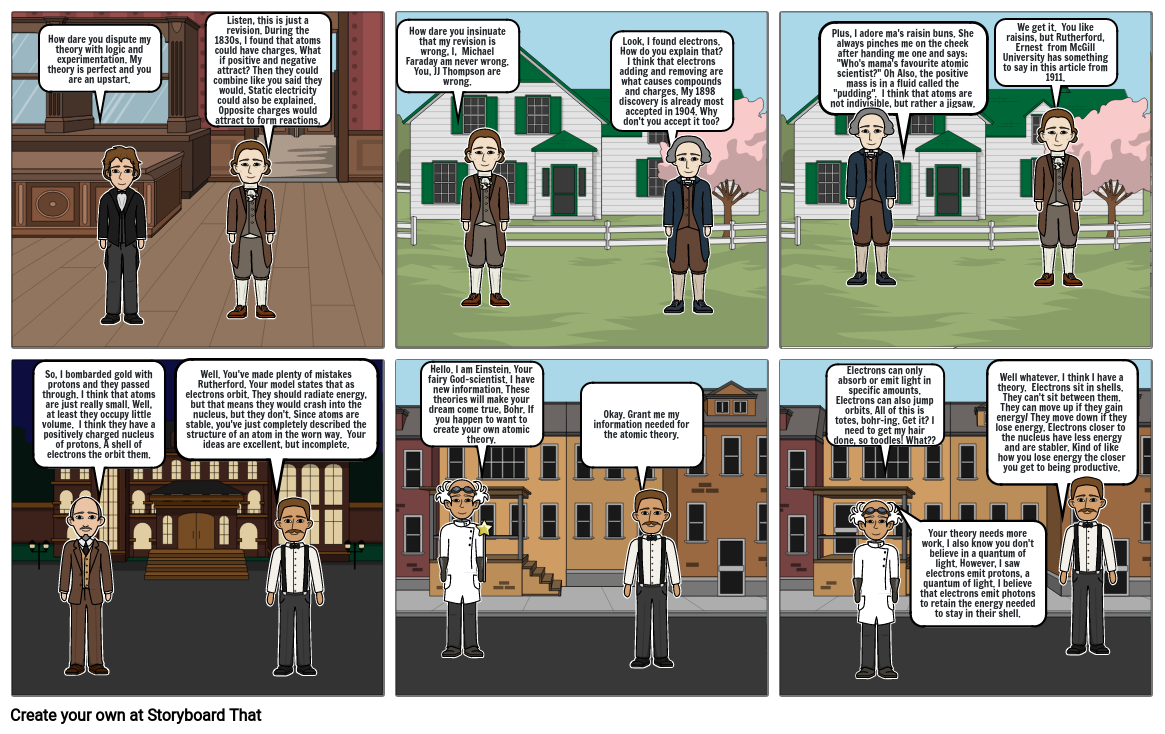
- How dare you dispute my theory with logic and experimentation. My theory is perfect and you are an upstart.
- Listen, this is just a revision. During the 1830s, I found that atoms could have charges. What if positive and negative attract? Then they could combine like you said they would. Static electricity could also be explained. Opposite charges would attract to form reactions,
- How dare you insinuate that my revision is wrong, I, Michael Faraday am never wrong. You, JJ Thompson are wrong.
- Look, I found electrons. How do you explain that? I think that electrons adding and removing are what causes compounds and charges. My 1898 discovery is already most accepted in 1904. Why don't you accept it too?
- Plus, I adore ma's raisin buns. She always pinches me on the cheek after handing me one and says: "Who's mama's favourite atomic scientist?" Oh Also, the positive mass is in a fluid called the "pudding". I think that atoms are not indivisible, but rather a jigsaw.
- We get it. You like raisins, but Rutherford, Ernest from McGill University has something to say in this article from 1911.
- So, I bombarded gold with protons and they passed through. I think that atoms are just really small. Well, at least they occupy little volume. I think they have a positively charged nucleus of protons. A shell of electrons the orbit them.
- Well. You've made plenty of mistakes Rutherford. Your model states that as electrons orbit. They should radiate energy, but that means they would crash into the nucleus, but they don't. Since atoms are stable, you've just completely described the structure of an atom in the worn way. Your ideas are excellent, but incomplete.
- Hello. I am Einstein. Your fairy God-scientist. I have new information. These theories will make your dream come true, Bohr. If you happen to want to create your own atomic theory.
- Okay. Grant me my information needed for the atomic theory.
- Electrons can only absorb or emit light in specific amounts. Electrons can also jump orbits. All of this is totes, bohr-ing. Get it? I need to get my hair done, so toodles! What??
- Your theory needs more work. I also know you don't believe in a quantum of light. However, I saw electrons emit protons, a quantum of light. I believe that electrons emit photons to retain the energy needed to stay in their shell.
- Well whatever. I think I have a theory. Electrons sit in shells. They can't sit between them. They can move up if they gain energy/ They move down if they lose energy. Electrons closer to the nucleus have less energy and are stabler. Kind of like how you lose energy the closer you get to being productive.
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!
