Unknown Story
Storyboard Text
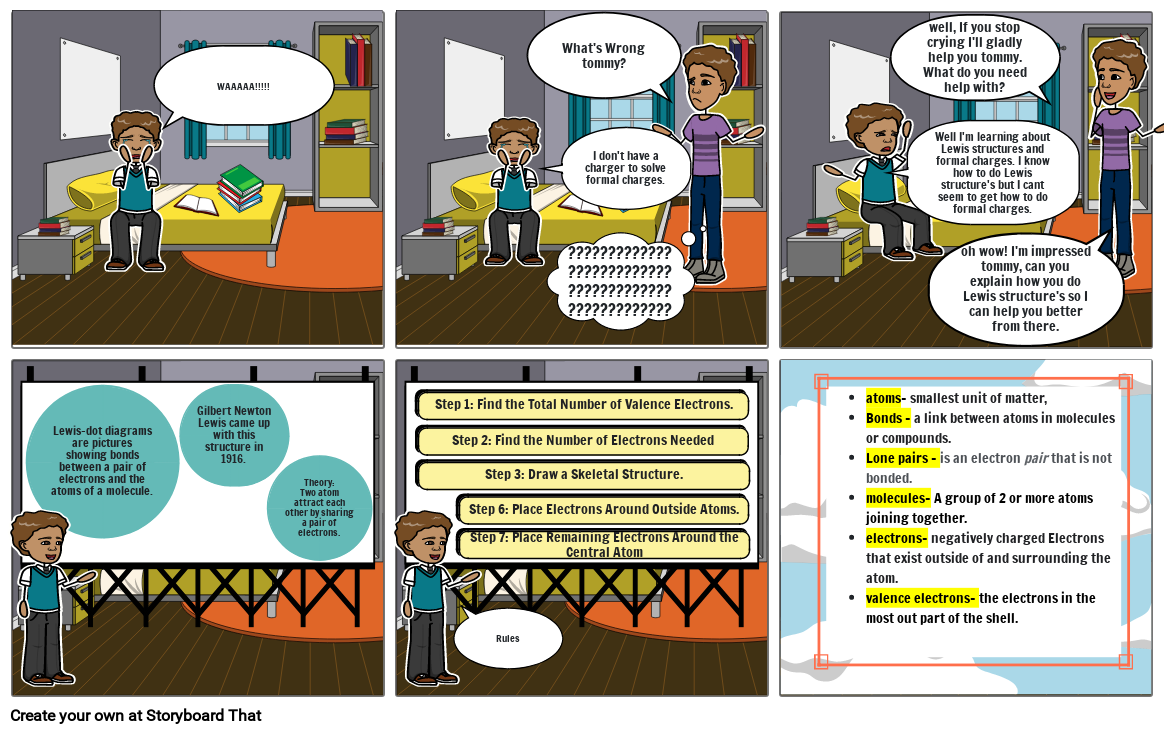
- WAAAAA!!!!!
- What's Wrong tommy?
- I don't have a charger to solve formal charges.
- ????????????????????????????????????????????????????
- Well I'm learning about Lewis structures and formal charges. I know how to do Lewis structure's but I cant seem to get how to do formal charges.
- well, If you stop crying I'll gladly help you tommy. What do you need help with?
- oh wow! I'm impressed tommy, can you explain how you do Lewis structure's so I can help you better from there.
- Lewis-dot diagrams are pictures showing bonds between a pair of electrons and the atoms of a molecule.
- Gilbert Newton Lewis came up with this structure in 1916.
- Theory:Two atom attract each other by sharing a pair of electrons.
- Step 1: Find the Total Number of Valence Electrons.
- Step 2: Find the Number of Electrons Needed
- Step 3: Draw a Skeletal Structure.
- Rules
- Step 6: Place Electrons Around Outside Atoms.
- Step 7: Place Remaining Electrons Around the Central Atom
- atoms- smallest unit of matter,Bonds - a link between atoms in molecules or compounds.Lone pairs - is an electron pair that is not bonded.molecules- A group of 2 or more atoms joining together.electrons- negatively charged Electrons that exist outside of and surrounding the atom.valence electrons- the electrons in the most out part of the shell.
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!
