Molecular Biology
Storyboard Text
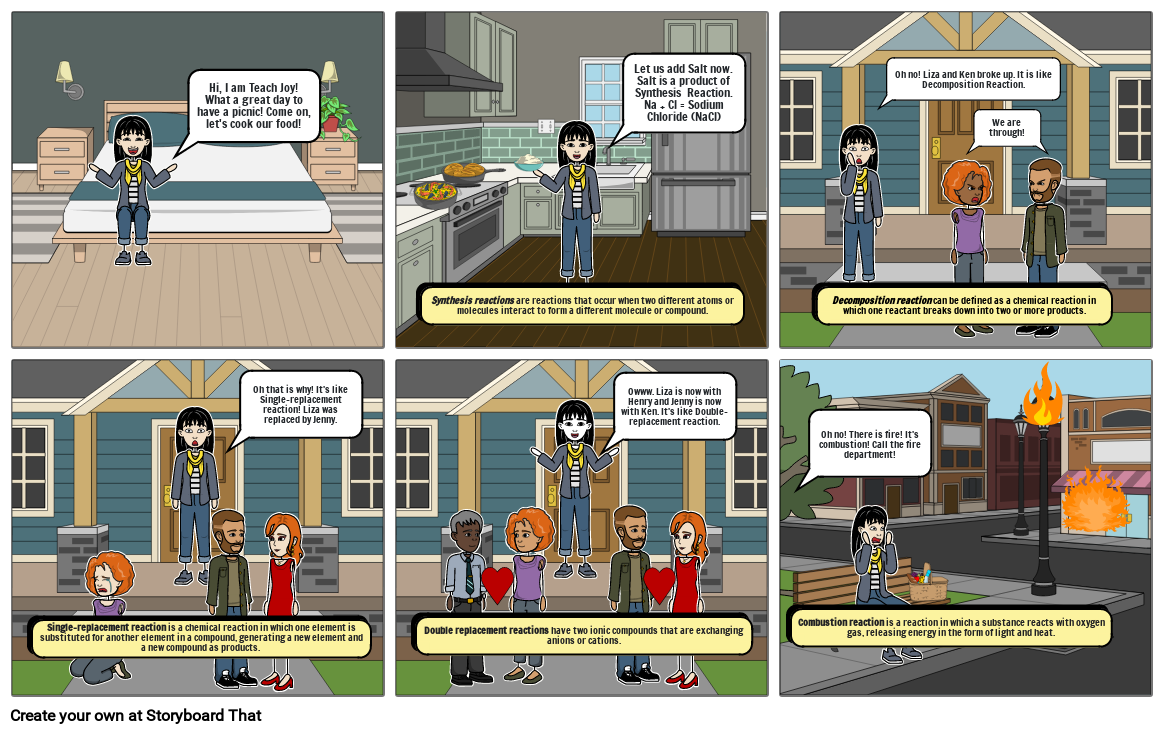
- Hi, I am Teach Joy!What a great day to have a picnic! Come on, let's cook our food!
- Synthesis reactions arereactions that occur when two different atoms or molecules interact to form a different molecule or compound.
- Let us add Salt now. Salt is a product of Synthesis Reaction. Na + Cl = Sodium Chloride (NaCl)
- Decomposition reactioncan be defined as a chemical reaction in which one reactant breaks down into two or more products.
- Oh no! Liza and Ken broke up. It is like Decomposition Reaction.
- We are through!
- Single-replacement reaction is a chemical reaction in which one element is substituted for another element in a compound, generating a new element and a new compound as products.
- Oh that is why! It's like Single-replacement reaction! Liza was replaced by Jenny.
- Double replacement reactions havetwo ionic compounds that are exchanging anions or cations.
- Owww. Liza is now with Henry and Jenny is now with Ken. It's like Double-replacement reaction.
- Combustion reaction isa reaction in which a substance reacts with oxygen gas, releasing energy in the form of light and heat.
- Oh no! There is fire! It's combustion! Call the fire department!
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!


