Atomic theory
Storyboard Text
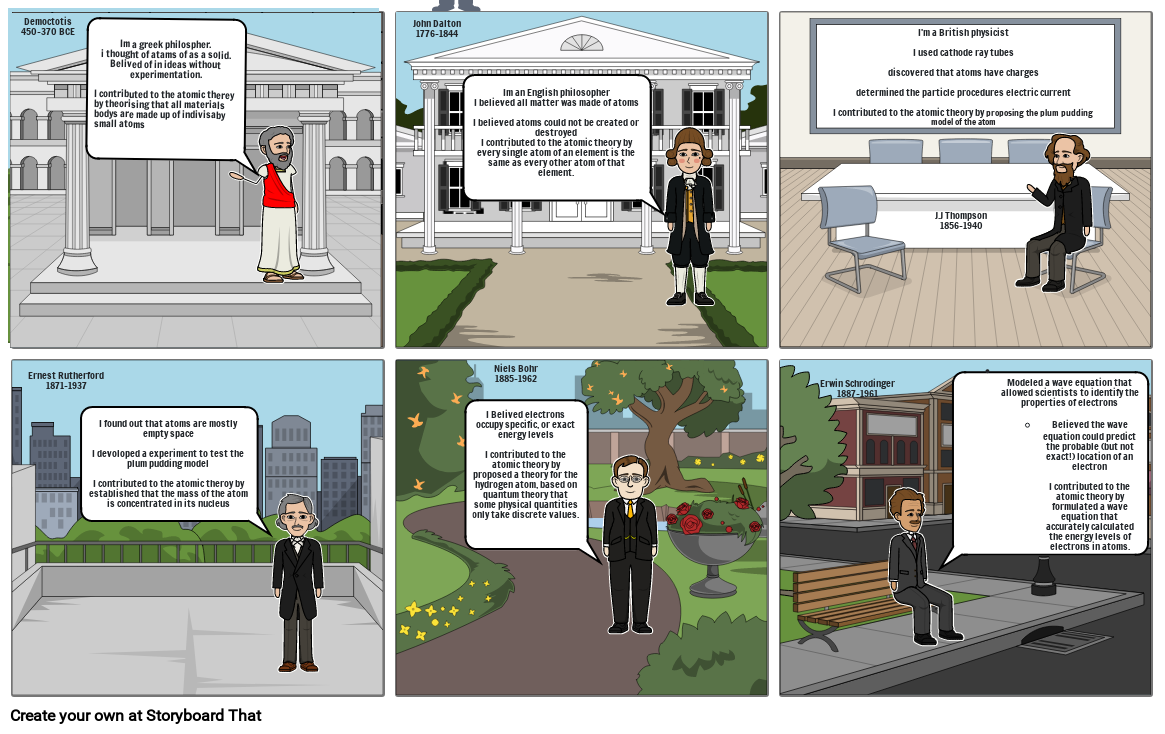
- Democtotis 450-370 BCE
- Ernest Rutherford1871-1937
- Im a greek philospher.i thought of atams of as a solid.Belived of in ideas without experimentation.I contributed to the atomic therey by theorising that all materials bodys are made up of indivisaby small atoms
- John Dalton1776-1844
- Im an English philosopherI believed all matter was made of atomsI believed atoms could not be created or destroyedI contributed to the atomic theory by every single atom of an element is the same as every other atom of that element.
- Niels Bohr1885-1962
- Erwin Schrodinger1887-1961
- I'm a British physicistI used cathode ray tubesdiscovered that atoms have chargesdetermined the particle procedures electric currentI contributed to the atomic theory by proposing the plum pudding model of the atom
- J.J Thompson1856-1940
- I found out that atoms are mostly empty spaceI devoloped a experiment to test the plum pudding modelI contributed to the atomic theroy by established that the mass of the atom is concentrated in its nucleus
- I Belived electrons occupy specific, or exact energy levelsI contributed to the atomic theory by proposed a theory for the hydrogen atom, based on quantum theory that some physical quantities only take discrete values.
- Modeled a wave equation that allowed scientists to identify the properties of electrons Believed the wave equation could predict the probable (but not exact!) location of an electronI contributed to the atomic theory by formulated a wave equation that accurately calculated the energy levels of electrons in atoms.
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!
