Atomic Model's
Storyboard Text
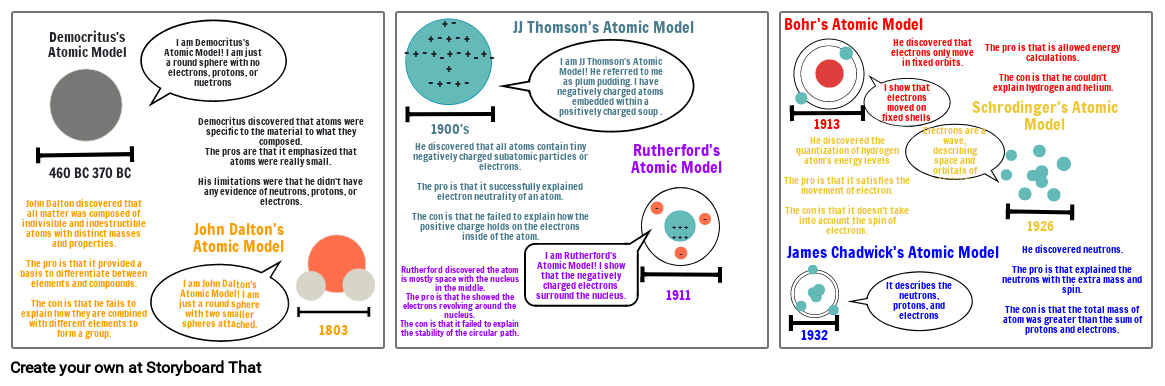
- 460 BC 370 BC
- John Dalton discovered that all matter was composed of indivisible and indestructible atoms with distinct masses and properties. The pro is that it provided a basis to differentiate between elements and compounds.The con is that he fails to explain how they are combined with different elements to form a group.
- Democritus's Atomic Model
- I am Democritus's Atomic Model! I am just a round sphere with no electrons, protons, or nuetrons
- I am John Dalton's Atomic Model! I am just a round sphere with two smaller spheres attached.
- John Dalton's Atomic Model
- Democritus discovered that atoms were specific to the material to what they composed. The pros are that it emphasized that atoms were really small.His limitations were that he didn't have any evidence of neutrons, protons, or electrons.
- 1803
- + - + - + - +- + - + _ + - + _ + _ +- + - + -
- 1900's
- Rutherford discovered the atom is mostly space with the nucleus in the middle.The pro is that he showed the electrons revolving around the nucleus.The con is that it failed to explain the stability of the circular path.
- He discovered that all atoms contain tiny negatively charged subatomic particles or electrons.The pro is that it successfully explained electron neutrality of an atom.The con is that he failed to explain how the positive charge holds on the electrons inside of the atom.
- JJ Thomson's Atomic Model
- I am JJ Thomson's Atomic Model! He referred to me as plum pudding. I have negatively charged atoms embedded within a positively charged soup .
- I am Rutherford's Atomic Model! I show that the negatively charged electrons surround the nucleus.
- Rutherford's Atomic Model
- -
- 1911
- -
- + + + + + +
- -
- James Chadwick's Atomic Model
- Bohr's Atomic Model
- 1932
- 1913
- He discovered the quantization of hydrogen atom's energy levelsThe pro is that it satisfies the movement of electron.The con is that it doesn't take into account the spin of electrons.
- It describes the neutrons, protons, and electrons
- I show that electrons moved on fixed shells
- He discovered that electrons only move in fixed orbits.
- Electrons are a wave, describing space and orbitals of electrons.
- Schrodinger's Atomic Model
- The pro is that is allowed energy calculations.The con is that he couldn't explain hydrogen and helium.
- 1926
- He discovered neutrons.The pro is that explained the neutrons with the extra mass and spin.The con is that the total mass of atom was greater than the sum of protons and electrons.
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!
