Discovery of the Periodic Table
Storyboard Text
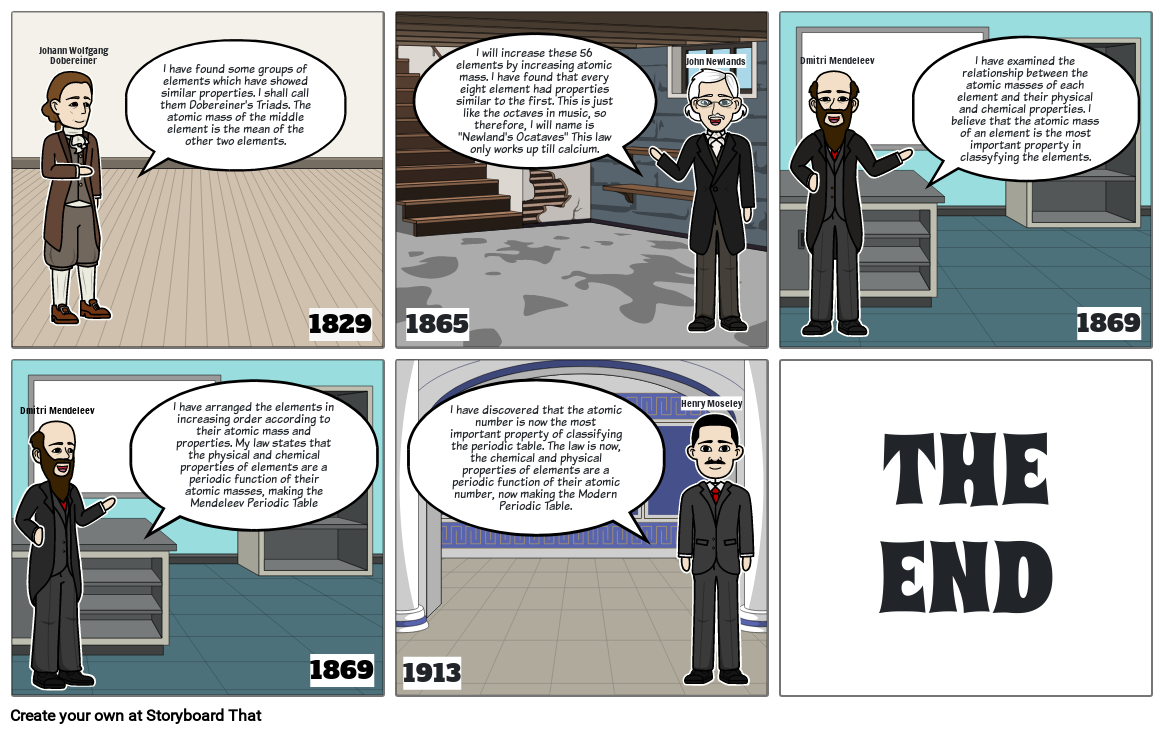
- Johann Wolfgang Dobereiner
- I have found some groups of elements which have showed similar properties. I shall call them Dobereiner's Triads. The atomic mass of the middle element is the mean of the other two elements.
- 1829
- 1865
- I will increase these 56 elements by increasing atomic mass. I have found that every eight element had properties similar to the first. This is just like the octaves in music, so therefore, I will name is Newland's Ocataves This law only works up till calcium.
- John Newlands
- Dmitri Mendeleev
- I have examined the relationship between the atomic masses of each element and their physical and chemical properties. I believe that the atomic mass of an element is the most important property in classyfying the elements.
- 1869
- Dmitri Mendeleev
- I have arranged the elements in increasing order according to their atomic mass and properties. My law states that the physical and chemical properties of elements are a periodic function of their atomic masses, making the Mendeleev Periodic Table
- 1869
- 1913
- I have discovered that the atomic number is now the most important property of classifying the periodic table. The law is now, the chemical and physical properties of elements are a periodic function of their atomic number, now making the Modern Periodic Table.
- Henry Moseley
- THEEND
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!


