ivann atomic theory
Storyboard Text
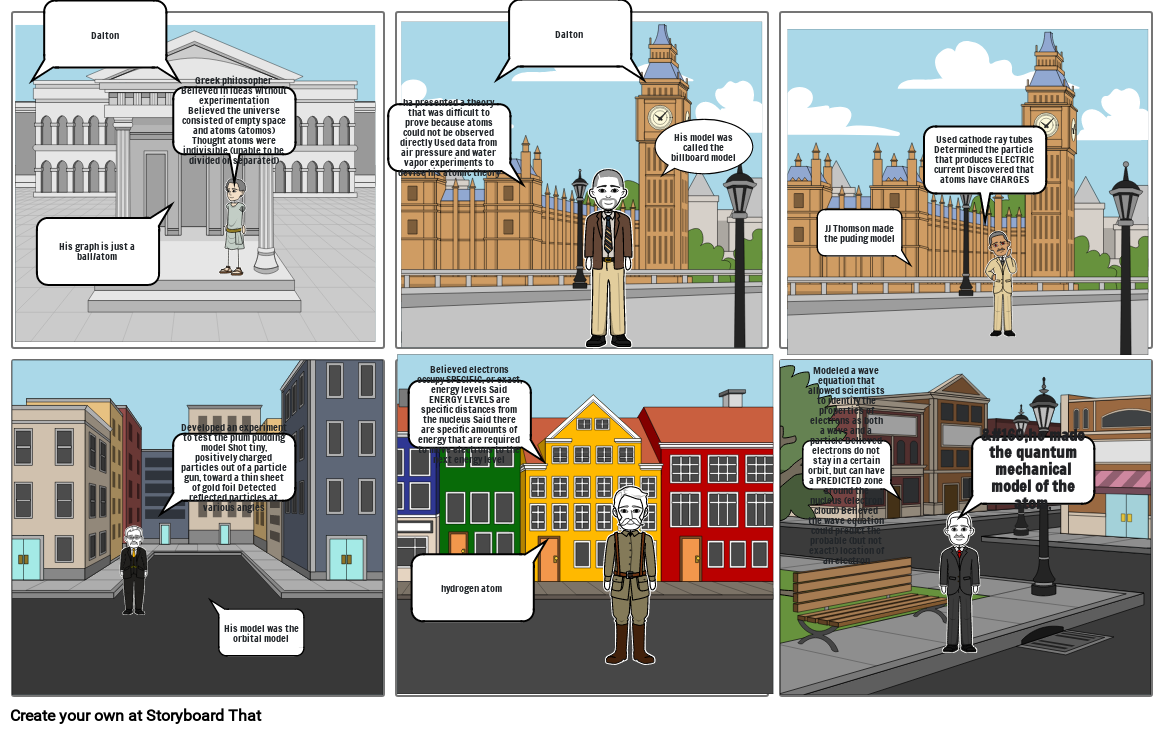
- Dalton
- His graph is just a ball/atom
- Greek philosopher Believed in ideas without experimentation Believed the universe consisted of empty space and atoms (atomos) Thought atoms were indivisible (unable to be divided or separated)
- ha presented a theory that was difficult to prove because atoms could not be observed directly Used data from air pressure and water vapor experiments to devise his atomic theory
- Dalton
- His model was called the billboard model
- JJ Thomson made the puding model
- Used cathode ray tubes Determined the particle that produces ELECTRIC current Discovered that atoms have CHARGES
- Developed an experiment to test the plum pudding model Shot tiny, positively charged particles out of a particle gun, toward a thin sheet of gold foil Detected reflected particles at various angles
- His model was the orbital model
- Believed electrons occupy SPECIFIC, or exact, energy levels Said ENERGY LEVELS are specific distances from the nucleus Said there are specific amounts of energy that are required to move electrons to the next energy level
- hydrogen atom
- Modeled a wave equation that allowed scientists to identify the properties of electrons as both a wave and a particle Believed electrons do not stay in a certain orbit, but can have a PREDICTED zone around the nucleus (electron cloud) Believed the wave equation could predict the probable (but not exact!) location of an electron
- he made the quantum mechanical model of the atom,
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!
