Chemical Bonding Project
Storyboard Text
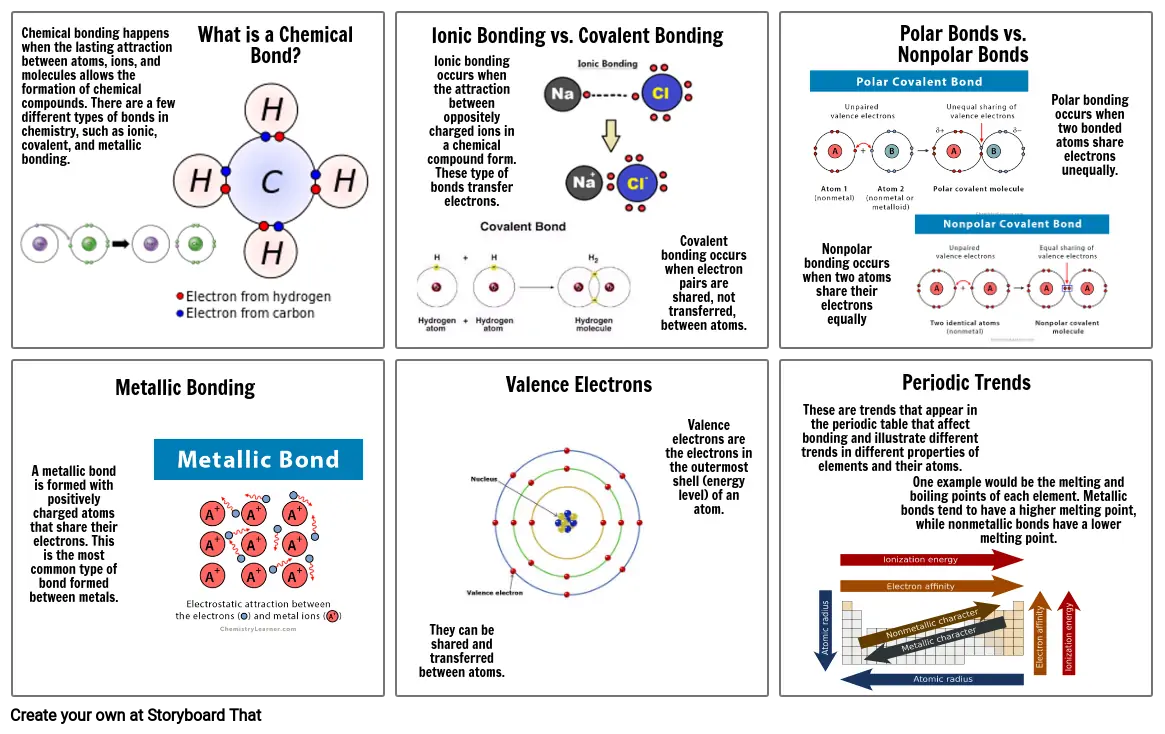
- Chemical bonding happens when the lasting attraction between atoms, ions, and molecules allows the formation of chemical compounds. There are a few different types of bonds in chemistry, such as ionic, covalent, and metallic bonding.
- Metallic Bonding
- What is a Chemical Bond?
- Ionic Bonding vs. Covalent Bonding
- Ionic bonding occurs when the attraction between oppositely charged ions in a chemical compound form. These type of bonds transfer electrons.
- Valence Electrons
- Covalent bonding occurs when electron pairs are shared, not transferred, between atoms.
- Nonpolar bonding occurs when two atoms share their electrons equally
- Polar Bonds vs. Nonpolar Bonds
- Periodic Trends
- Polar bonding occurs when two bonded atoms share electrons unequally.
- A metallic bond is formed with positively charged atoms that share their electrons. This is the most common type of bond formed between metals.
- They can be shared and transferred between atoms.
- Valence electrons are the electrons in the outermost shell (energy level) of an atom.
- These are trends that appear in the periodic table that affect bonding and illustrate different trends in different properties of elements and their atoms.
- One example would be the melting and boiling points of each element. Metallic bonds tend to have a higher melting point, while nonmetallic bonds have a lower melting point.
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!
