Unknown Story
Storyboard Text
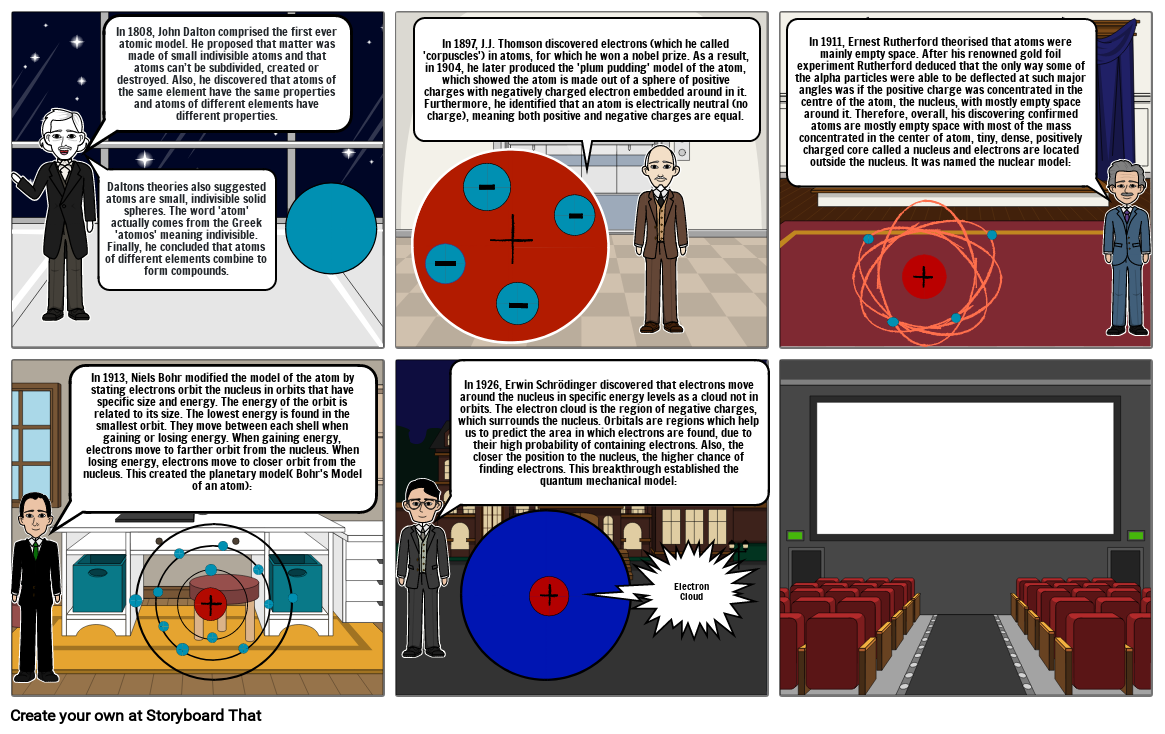
- In 1808, John Dalton comprised the first ever atomic model. He proposed that matter was made of small indivisible atoms and that atoms can’t be subdivided, created or destroyed. Also, he discovered that atoms of the same element have the same properties and atoms of different elements have different properties.
- Daltons theories also suggested atoms are small, indivisible solid spheres. The word 'atom' actually comes from the Greek 'atomos' meaning indivisible. Finally, he concluded that atoms of different elements combine to form compounds.
- In 1897, J.J. Thomson discovered electrons (which he called 'corpuscles') in atoms, for which he won a nobel prize. As a result, in 1904, he later produced the 'plum pudding' model of the atom, which showed the atom is made out of a sphere of positive charges with negatively charged electron embedded around in it. Furthermore, he identified that an atom is electrically neutral (no charge), meaning both positive and negative charges are equal.
- In 1911, Ernest Rutherford theorised that atoms were mainly empty space. After his renowned gold foil experiment Rutherford deduced that the only way some of the alpha particles were able to be deflected at such major angles was if the positive charge was concentrated in the centre of the atom, the nucleus, with mostly empty space around it. Therefore, overall, his discovering confirmed atoms are mostly empty space with most of the mass concentrated in the center of atom, tiny, dense, positively charged core called a nucleus and electrons are located outside the nucleus. It was named the nuclear model:
- In 1913, Niels Bohr modified the model of the atom by stating electrons orbit the nucleus in orbits that have specific size and energy. The energy of the orbit is related to its size. The lowest energy is found in the smallest orbit. They move between each shell when gaining or losing energy. When gaining energy, electrons move to farther orbit from the nucleus. When losing energy, electrons move to closer orbit from the nucleus. This created the planetary model( Bohr's Model of an atom):
- In 1926, Erwin Schrödinger discovered that electrons move around the nucleus in specific energy levels as a cloud not in orbits. The electron cloud is the region of negative charges, which surrounds the nucleus. Orbitals are regions which help us to predict the area in which electrons are found, due to their high probability of containing electrons. Also, the closer the position to the nucleus, the higher chance of finding electrons. This breakthrough established the quantum mechanical model:
- Electron Cloud
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!
