chemistry
Storyboard Text
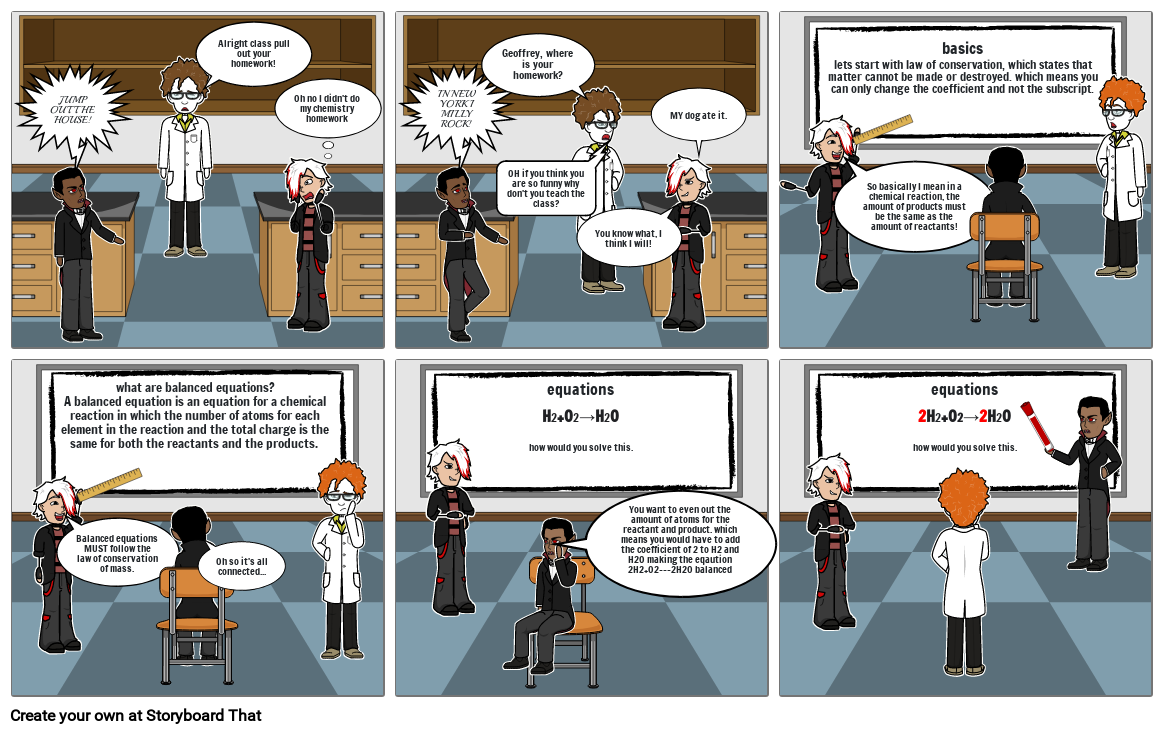
- JUMP OUT THE HOUSE!
- Alright class pull out your homework!
- Oh no I didn't do my chemistry homework
- IN NEW YORK I MILLY ROCK!
- Geoffrey, where is your homework?
- OH if you think you are so funny why don't you teach the class?
- You know what, I think I will!
- MY dog ate it.
- basicslets start with law of conservation, which states that matter cannot be made or destroyed. which means you can only change the coefficient and not the subscript.
- So basically I mean in a chemical reaction, the amount of products must be the same as the amount of reactants!
- what are balanced equations?A balanced equation is an equation for a chemical reaction in which the number of atoms for each element in the reaction and the total charge is the same for both the reactants and the products.
- Balanced equations MUST follow the law of conservation of mass.
- Oh so it's all connected...
- equationsH2+O2→H2Ohow would you solve this.
- You want to even out the amount of atoms for the reactant and product. which means you would have to add the coefficient of 2 to H2 and H2O making the eqaution 2H2+O2--->2H20 balanced
- equations2H2+O2→2H2Ohow would you solve this.
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!
