matter
Storyboard Text
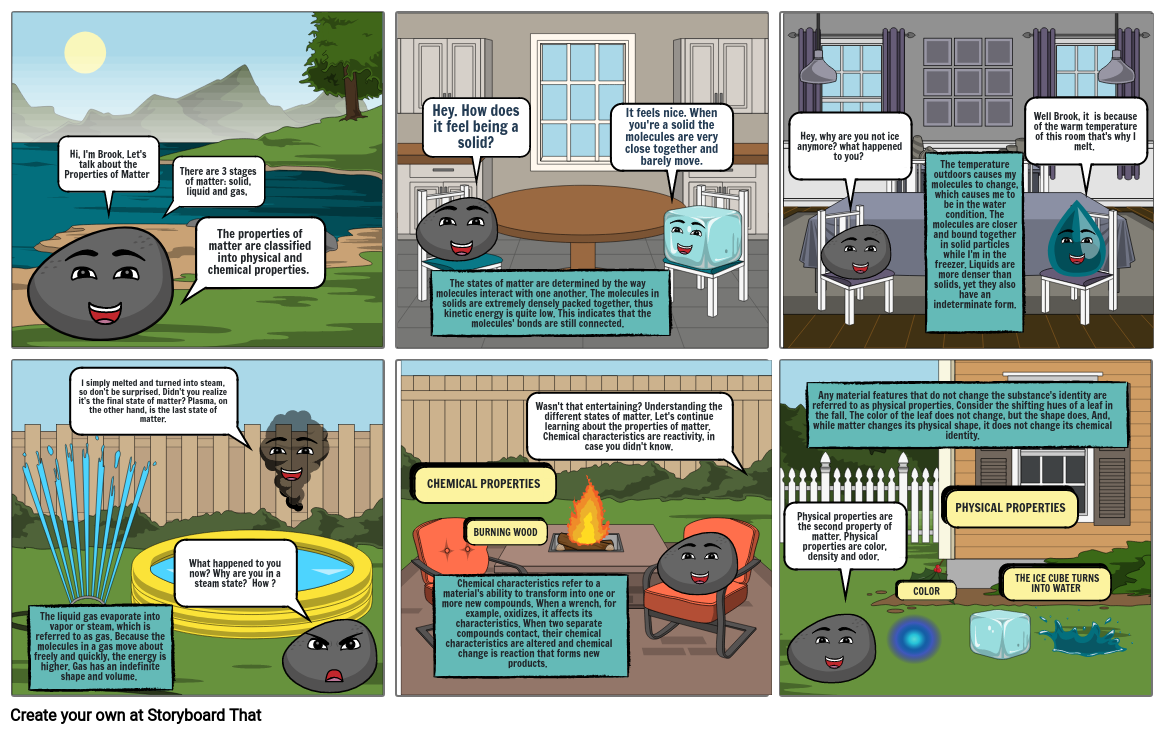
- Hi, I'm Brook. Let's talk about the Properties of Matter
- There are 3 stages of matter: solid, liquid and gas,
- The properties of matter are classified into physical and chemical properties.
- Hey. How does it feel being a solid?
- The states of matter are determined by the way molecules interact with one another. The molecules in solids are extremely densely packed together, thus kinetic energy is quite low. This indicates that the molecules' bonds are still connected.
- It feels nice. When you're a solid the molecules are very close together and barely move.
- Hey, why are you not ice anymore? what happened to you?
- The temperature outdoors causes my molecules to change, which causes me to be in the water condition. The molecules are closer and bound together in solid particles while I'm in the freezer. Liquids are more denser than solids, yet they also have an indeterminate form.
- Well Brook, it is because of the warm temperature of this room that's why I melt.
- The liquid gas evaporate into vapor or steam, which is referred to as gas. Because the molecules in a gas move about freely and quickly, the energy is higher. Gas has an indefinite shape and volume.
- I simply melted and turned into steam, so don't be surprised. Didn't you realize it's the final state of matter? Plasma, on the other hand, is the last state of matter.
- What happened to you now? Why are you in a steam state? How ?
- CHEMICAL PROPERTIES
- Chemical characteristics refer to a material's ability to transform into one or more new compounds. When a wrench, for example, oxidizes, it affects its characteristics. When two separate compounds contact, their chemical characteristics are altered and chemical change is reaction that forms new products.
- BURNING WOOD
- Wasn't that entertaining? Understanding the different states of matter. Let's continue learning about the properties of matter. Chemical characteristics are reactivity, in case you didn't know.
- Physical properties are the second property of matter. Physical properties are color, density and odor.
- Any material features that do not change the substance's identity are referred to as physical properties. Consider the shifting hues of a leaf in the fall. The color of the leaf does not change, but the shape does. And, while matter changes its physical shape, it does not change its chemical identity.
- COLOR
- PHYSICAL PROPERTIES
- THE ICE CUBE TURNS INTO WATER
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!


