The Development of the Modern Atom
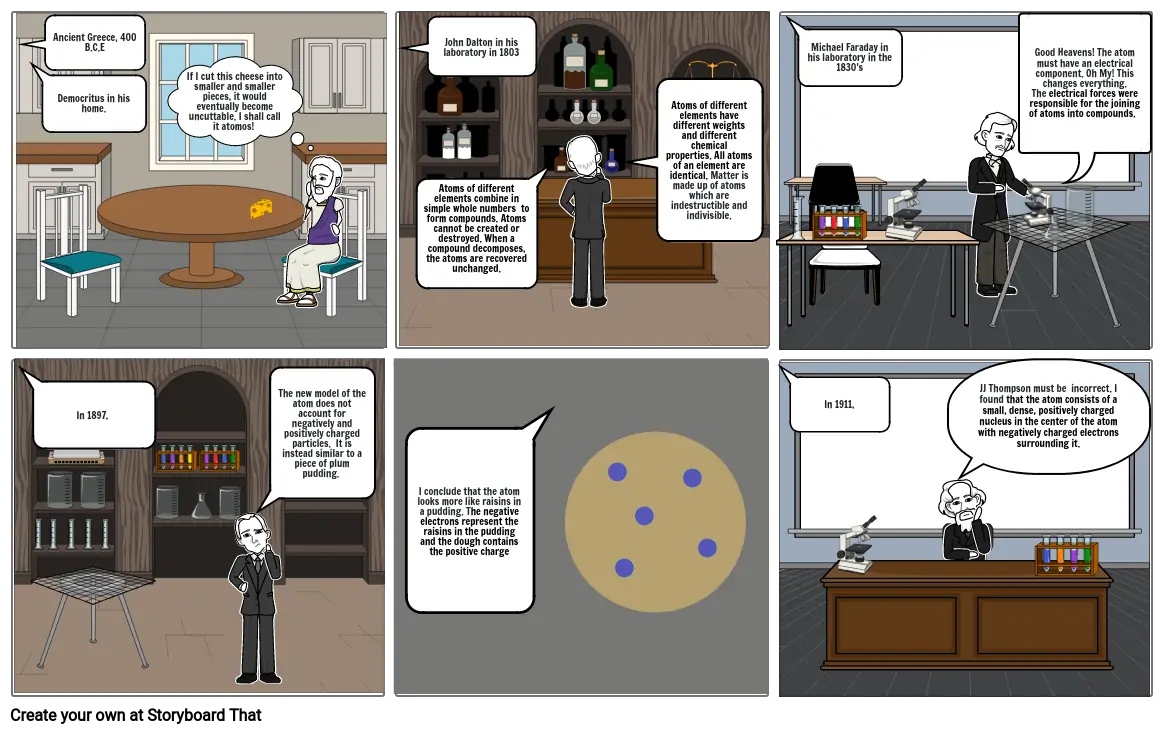
Storyboard Text
- Ancient Greece, 400 B.C.E
- Democritus in his home.
- If I cut this cheese into smaller and smaller pieces, it would eventually become uncuttable. I shall call it atomos!
- John Dalton in his laboratory in 1803
- Atoms of different elements combine in simple whole numbers to form compounds. Atoms cannot be created or destroyed. When a compound decomposes, the atoms are recovered unchanged.
- Atoms of different elements have different weights and different chemical properties. All atoms of an element are identical. Matter is made up of atoms which are indestructible and indivisible.
- Michael Faraday in his laboratory in the 1830's
- Good Heavens! The atom must have an electrical component. Oh My! This changes everything. The electrical forces were responsible for the joining of atoms into compounds.
- In 1897,
-
- The new model of the atom does not account for negatively and positively charged particles. It is instead similar to a piece of plum pudding.
-
- I conclude that the atom looks more like raisins in a pudding. The negative electrons represent the raisins in the pudding and the dough contains the positive charge
-
-
-
-
-
-
- In 1911,
- JJ Thompson must be incorrect. I found that the atom consists of a small, dense, positively charged nucleus in the center of the atom with negatively charged electrons surrounding it.
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!
