Storytime science
Storyboard Text
- CATION
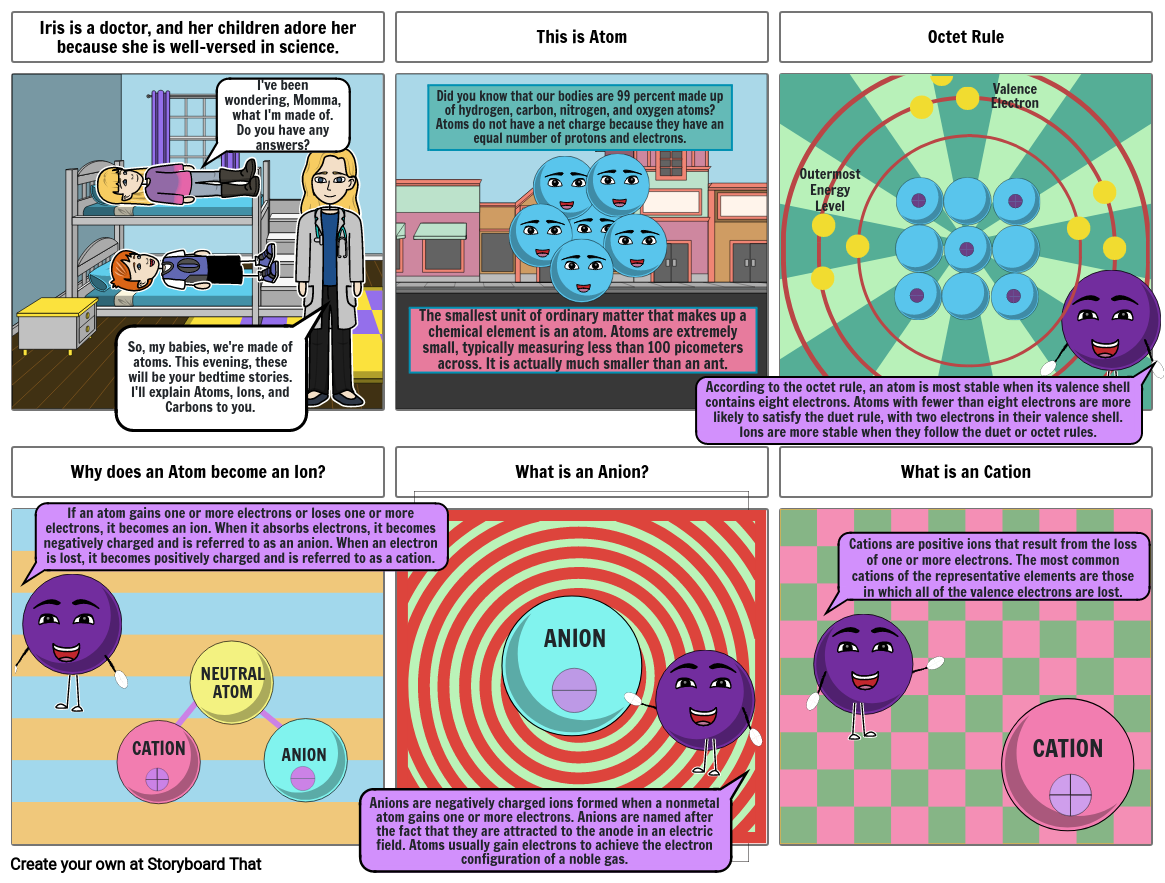
- Iris is a doctor, and her children adore her because she is well-versed in science.
- NEUTRALATOM
- So, my babies, we're made of atoms. This evening, these will be your bedtime stories. I'll explain Atoms, Ions, and Carbons to you.
- ANION
- I've been wondering, Momma, what I'm made of. Do you have any answers?
- This is Atom
- The smallest unit of ordinary matter that makes up a chemical element is an atom. Atoms are extremely small, typically measuring less than 100 picometers across. It is actually much smaller than an ant.
- Did you know that our bodies are 99 percent made up of hydrogen, carbon, nitrogen, and oxygen atoms? Atoms do not have a net charge because they have an equal number of protons and electrons.
- Octet Rule
- OutermostEnergyLevel
- Valence Electron
- Why does an Atom become an Ion?
- If an atom gains one or more electrons or loses one or more electrons, it becomes an ion. When it absorbs electrons, it becomes negatively charged and is referred to as an anion. When an electron is lost, it becomes positively charged and is referred to as a cation.
- What is an Anion?
- ANION
- According to the octet rule, an atom is most stable when its valence shell contains eight electrons. Atoms with fewer than eight electrons are more likely to satisfy the duet rule, with two electrons in their valence shell. Ions are more stable when they follow the duet or octet rules.
- What is an Cation
- Cations are positive ions that result from the loss of one or more electrons. The most common cations of the representative elements are those in which all of the valence electrons are lost.
- Anions are negatively charged ions formed when a nonmetal atom gains one or more electrons. Anions are named after the fact that they are attracted to the anode in an electric field. Atoms usually gain electrons to achieve the electron configuration of a noble gas.
- CATION
Over 30 Million Storyboards Created
No Downloads, No Credit Card, and No Login Needed to Try!


